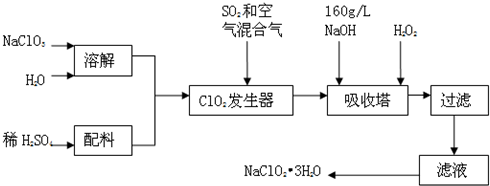
��Ŀ����
��1��1L Na2SO4 ��Һ�У��������Ԫ�ص�������2.3g����Һ�� SO42-���ʵ���Ũ��Ϊ
��2�����ʵ����û�� Na2SO4������ NaOH �� H2SO4 ��Ӧ���� Na2SO4 ��Һ���������ϼ��㣬���Ʒ��ϣ�1����Ҫ��� Na2SO4 ��Һ����ҪNaOH������Ϊ
0.05
0.05
mol/L����2�����ʵ����û�� Na2SO4������ NaOH �� H2SO4 ��Ӧ���� Na2SO4 ��Һ���������ϼ��㣬���Ʒ��ϣ�1����Ҫ��� Na2SO4 ��Һ����ҪNaOH������Ϊ
4
4
g �� 1.0mol/L������Һ�����Ϊ50
50
mL����˵����������������Ϻ�������ˮϡ����1L ���ɣ���������1�������Ƶ����������Ƶ����ʵ���������C=
���������ӵ����ʵ���Ũ�ȣ���������Һ��������Ũ�������������Ũ��֮����2��1��
��2��������ԭ���غ�����������Ƶ���������������������غ��������������
| n |
| V |
��2��������ԭ���غ�����������Ƶ���������������������غ��������������
����⣺��1�������ӵ����ʵ���=
=0.1mol���������ӵ����ʵ���Ũ��=
=0.1mol/L����������Һ��������Ũ�������������Ũ��֮����2��1������SO42-���ʵ���Ũ��=0.1mol/L��0.5=0.05mol/L��
�ʴ�Ϊ��0.05��
��2�������������غ�֪���������Ƶ�����=
��40g/mol=4g��
��������������غ�ã���������ʵ���=
=0.05L=50mL��
�ʴ�Ϊ��4��50��
| 2.3g |
| 23g/mol |
| 0.1mol |
| 1L |
�ʴ�Ϊ��0.05��
��2�������������غ�֪���������Ƶ�����=
| 2.3g |
| 23g/mol |
��������������غ�ã���������ʵ���=
| 0.1mol/L��0.5��1L |
| 1.0mol/L |
�ʴ�Ϊ��4��50��
���������⿼�������ʵ���Ũ�ȵ��йؼ��㣬��ȷ���ʼ�Ĺ�ϵ�ǽⱾ��ؼ�������ԭ���غ���������ɣ��ѶȲ���

��ϰ��ϵ�д�
 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д�
�����Ŀ
 HS-+OH-
HS-+OH-