��Ŀ����
��ͼ��ʾװ�ã�A��B�е�Ϊ��Ķ��Ե缫��C��DΪ����ʪ��![]() ��ֽ���ϵIJ��У���Դ��a��b����������A��B�г���
��ֽ���ϵIJ��У���Դ��a��b����������A��B�г���![]() ��Һ������ʢ��
��Һ������ʢ��![]() ��Һ��ˮ���У��ж�
��Һ��ˮ���У��ж�![]() ���պ�
���պ�![]() ��ֱͨ���磮��
��ֱͨ���磮��
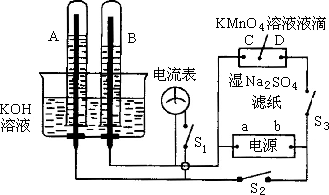
(1)�����Դ����������aΪ________����bΪ________����
(2)��ʪ��![]() ��Һ��ֽ�����ĵ�
��Һ��ֽ�����ĵ�![]() Һ�Σ�������������________________��
Һ�Σ�������������________________��
(3)д���缫��Ӧʽ��A��____________��B��_______________��
(4)�����һ��ʱ���A��B�о������ݰ�Χ�缫����ʱ�ж�![]() ���պ�
���պ�![]() �����������ָ��________(��ᡱ���ᡱ)�ƶ��������ǣ�________________��
�����������ָ��________(��ᡱ���ᡱ)�ƶ��������ǣ�________________��
�𰸣�
������
��ʾ��
������
|
(1)��,�� (2)��ɫ��D�����ƶ� (3) (4)��,�������ȼ�ϵ�� |
��ʾ��
|
�ж� |

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ijС��ͬѧ��ʵ��������װ��ͼ��ʾװ�ã�aΪ��Ƭ��bΪʯī����ش�
ijС��ͬѧ��ʵ��������װ��ͼ��ʾװ�ã�aΪ��Ƭ��bΪʯī����ش�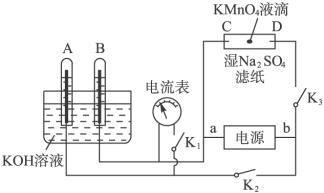
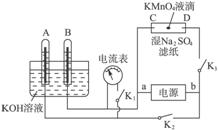
 Fe��Cu��Al�dz����Ľ���Ԫ�أ���Ҫ��ش��������⣺
Fe��Cu��Al�dz����Ľ���Ԫ�أ���Ҫ��ش��������⣺