��Ŀ����
�й��������������Դ�ḻ���ǽ���ұ���ͼӹ�����Ҫ����֮һ��ij��ʢ���Ļ�ͭ��( CuFeS2)���Ʊ�ͭ���仯�������Ҫԭ��֮һ�������Ʊ������Ļ����
(1)ұ��ͭ�ķ�ӦΪ8CuFeS2+21O2 8Cu +4FeO+2Fe2O3+16SO2����CuFeS2����Fe�Ļ��ϼ�Ϊ+2�ۣ���Ӧ�б���ԭ��Ԫ����______��
8Cu +4FeO+2Fe2O3+16SO2����CuFeS2����Fe�Ļ��ϼ�Ϊ+2�ۣ���Ӧ�б���ԭ��Ԫ����______��
(2)����ұ�������л��������SO2�����д���������������____��
A���߿��ŷ�
B�������Ʊ�����
C���ð�ˮ�����Ƶ���
D����Ũ��������
(3)���û�ͭ��ұ��ﵲ�����¯������Fe2O3��FeO��SiO2��Al2O3�����Ʊ�Fe2O3������Ϊ
����ϡ�����ȡ¯�������ˣ�
����Һ���������ټ������NaOH��Һ�����ˣ�������ϴ�ӡ�������յ�Fe2O3��
����������Ϣ�ش��������⣺
a����ȥAl3+�����ӷ���ʽ��____��
b��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO��
�ṩ�Լ�����Ũ�����ϡ�����KSCN��Һ��KMnO4��Һ��NaOH��Һ��ˮ
��ѡ�Լ�Ϊ___��֤��¯���к���FeO��ʵ������Ϊ____��
(4)¯���е�Fe2O3��ϡ�����ܽ�����ò�����е��Ļ�ѧ����ʽΪ____ ���缫Ϊ���Ե缫����
(5)��ȡmg����ұ�������Cu��FeO��Fe2O3����0.1 mol��L-1 500 mLϡ����ǡ����ȫ��Ӧ�����ܲ��������224 mL NO�������Ӧ�����Һ���ټ���0.2 mol��L-1 NaOH��Һ���պ�ʹ��Һ�н���������ȫ�������������NaOH��Һ____mL��
(1)Cu��O
(2)BC
(3)Al3+ +4OH-==AlO2-+2H2O�� �ڢܣ� ϡ�����ȡ¯��������ҺʹKMnO4��Һ��ɫ
(4)2Fe2(SO4)3 +2H2O 4FeSO4+O2��+2H2SO4
4FeSO4+O2��+2H2SO4
(5)200 mL

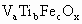 ����ʾ������V������5�ۣ�Ti�ԣ�4�ۣ�Fe�ԣ��ۣ���x��ֵ��_______(�ú�a��b��c�Ĵ���ʽ��ʾ)��
����ʾ������V������5�ۣ�Ti�ԣ�4�ۣ�Fe�ԣ��ۣ���x��ֵ��_______(�ú�a��b��c�Ĵ���ʽ��ʾ)��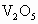 ��
��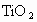 �����ʣ���д��
�����ʣ���д�� ��ijѧ����ȤС�����һ��ʵ��װ������������Ӧ���Ƶ�
��ijѧ����ȤС�����һ��ʵ��װ������������Ӧ���Ƶ� ��װ����β������������ͼ����ʾ��ƿ��Ӧ����_____________��Һ���ڵ��ܿ�B��Ӧ______________��
��װ����β������������ͼ����ʾ��ƿ��Ӧ����_____________��Һ���ڵ��ܿ�B��Ӧ______________��




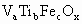 ����ʾ������V������5�ۣ�Ti�ԣ�4�ۣ�Fe�ԣ��ۣ���x��ֵ��_______(�ú�a��b��c�Ĵ���ʽ��ʾ)��
����ʾ������V������5�ۣ�Ti�ԣ�4�ۣ�Fe�ԣ��ۣ���x��ֵ��_______(�ú�a��b��c�Ĵ���ʽ��ʾ)��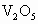 ��
��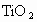 �����ʣ���д��
�����ʣ���д�� ��ijѧ����ȤС�����һ��ʵ��װ������������Ӧ���Ƶ�
��ijѧ����ȤС�����һ��ʵ��װ������������Ӧ���Ƶ�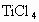 ��װ����β������������ͼ����ʾ��ƿ��Ӧ����_____________��Һ���ڵ��ܿ�B��Ӧ______________��
��װ����β������������ͼ����ʾ��ƿ��Ӧ����_____________��Һ���ڵ��ܿ�B��Ӧ______________��



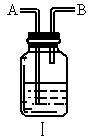
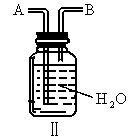
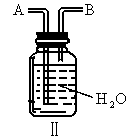
 TiCl4 +2CO,Cl2 +2NaOH = NaCl+ NaClO+ H2O��ij��ѧ��ȤС�����һ��ʵ��װ������������Ӧ���Ƶ�TiCl4��װ����β������������ͼ(I)��ʾ��ƿ��Ӧ����___��Һ���ڵ��ܿ�B��Ӧ___��
TiCl4 +2CO,Cl2 +2NaOH = NaCl+ NaClO+ H2O��ij��ѧ��ȤС�����һ��ʵ��װ������������Ӧ���Ƶ�TiCl4��װ����β������������ͼ(I)��ʾ��ƿ��Ӧ����___��Һ���ڵ��ܿ�B��Ӧ___�� 