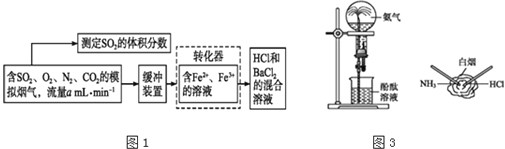
��Ŀ����
����Ŀ����ش��������⣺
(1)25��ʱ��pH=5��CH3COOH��Һ�У���������NaOH���壬����Һ��![]() _______(����������������С������������)��
_______(����������������������������)��
(2)25���£���ijNa2CO3��Һ�м���ϡ���ᣬ���к�̼Ԫ�صĸ��������ʵ�����������������ҺpH�仯�IJ��������ͼ��ʾ��

����ͬһ��Һ��,H2CO3��HCO3-��CO32-______�����������������������������档
����pH =7ʱ,��Һ�к�̼Ԫ�ص�����ҪΪ________����Һ�и������ӵ����ʵ���Ũ�ȴ�С��ϵΪ___________________________��
����Ӧ��CO32-+H2O![]() HCO3-+OH-��ƽ�ⳣ��KhֵΪ_________��
HCO3-+OH-��ƽ�ⳣ��KhֵΪ_________��
(3)������H2S��ԭ��Ϊ:H2S+Fe2(SO4)3=S��+2FeSO4+H2SO4��4FeSO4+O2+2H2SO4 2Fe2(SO4)3+2H2O��
2Fe2(SO4)3+2H2O��

����˾�����ʱ,FeSO4����������������ʱ��5��105�����þ���������___________��
����ͼ��ͼ���ж�ʹ����˾����������Ϊ____________������������£��÷�Ӧ�ļ���
��ʽΪ_________��
���𰸡����� ���� HCO3- c(Na+)>c(Cl-)>c(HCO3-)>c(H+)=c(OH-) 10-4 ���� 30�桢pH=2 ˮԡ����
��������
(1)���ݵ���ƽ�ⳣ��ֻ���¶��йط�����
(2)����ͼʾ�жϣ�pH=10ʱc(CO32-)=c(HCO3-) ���ݴ˼���CO32-+H2O![]() HCO3-+OH-��ƽ�ⳣ����
HCO3-+OH-��ƽ�ⳣ����
(3) ����˾�������˷�Ӧ���ʣ�����������£�Fe2+������������ߣ�
(1)�������ƽ�ⳣ��![]() ������ƽ�ⳣ��ֻ���¶��й� ������25��ʱ��pH=5��CH3COOH��Һ�м�������NaOH���壬
������ƽ�ⳣ��ֻ���¶��й� ������25��ʱ��pH=5��CH3COOH��Һ�м�������NaOH���壬![]() ��������Һ��
��������Һ��![]() ���䣻
���䣻
(2)�ٸ���ͼʾ����ͬһ��Һ�У�H2CO3��HCO3-��CO32-���ܴ������棻
�ڸ���ͼʾ����pH =7ʱ����Һ�к�̼Ԫ�ص�����ҪΪHCO3-������Na2CO3��Һ�м�������ʵ�����ϡ���ᣬ����̼�����ƺ��Ȼ��ƣ���ʱ��Һ�ʼ��ԣ��ټ��������ᣬ��ʹ��Һ�����ԣ���Һ�и������ӵ����ʵ���Ũ�ȴ�С��ϵΪc(Na+)>c(Cl-)>c(HCO3-)>c(H+)=c(OH-)��
pH=10ʱc(CO32-)=c(HCO3-) ��c(OH-)=10-4��![]() 10-4 ��
10-4 ��
(3) ����˾��������FeSO4�����������ʣ���˾��Ǵ�����
�ڸ���ͼʾ��30����pH=2ʱ��Fe2+������������ߣ�����ʹ����˾����������Ϊ30����pH=2�������¶�Ϊ30���ķ�����ˮԡ���ȡ�

����Ŀ����ͬ�¶��£��������ȵ����������ܱ������з������淴Ӧ��2SO3(g)![]() 2SO2(g)+O2(g)��H=+197kJ/mol��ʵ������ʼ��ƽ��ʱ���й��������±���
2SO2(g)+O2(g)��H=+197kJ/mol��ʵ������ʼ��ƽ��ʱ���й��������±���
������� | ��ʼʱ�������� �� ���� /mol | ƽ��ʱ��Ӧ�е������仯 | ||
SO3 | SO2 | O2 | ||
�� | 2 | 0 | 0 | ��������akJ |
�� | 0 | 2 | 1 | �ų��� �� bkJ |
�� | 4 | 0 | 0 | ��������ckJ |
����������ȷ���ǣ� ��
A. ��ƽ��ʱO2�������������>��
B. ������ϵ��aһ������b
C. �٢ڢ� ��Ӧ��ƽ�ⳣ������>��>��
D. ���е��ܶȲ��ٸı�ʱ˵����Ӧ�Ѵﵽƽ��״̬
����Ŀ��ijʵ��С���KSCN�����ʽ���̽�����������ʵ�飺
�Թ����Լ� | ʵ�� | �μ��Լ� | ���� |
KSCN��Һ | �� | i.�ȼ�1 mL 0.1 mol/L FeSO4��Һ ii.�ټ������ữ��KMnO4��Һ | i.���������� ii.�ȱ�죬����ɫ |
�� | iii.�ȵμ�1 mL 0.05 mol/L Fe2(SO4)3��Һ iv.�ٵμ�0.5 mL 0.5 mol/L FeSO4��Һ | iii.��Һ��� iv.��ɫ���Ա�dz |
(1)�������ӷ���ʽ��ʾʵ��I��Һ����ԭ��___________
�����ʵ��I�к�ɫ��ȥ��ԭ��С��ͬѧ��Ϊ��SCN������KMnO4����ΪSO42���������ͼʵ��װ��֤ʵ�˲����dz����ġ�

����X��Һ��_____________���������SO42�IJ�����������__________��
(2)���ʵ�������ɫ���Ա�dz����ʵ��С�����Ԥ�⡣
ԭ��٣�������ǿ����ʺ����������Ӽ�����ã�����֮��ǣ��������ǿ��������ЧӦ��������ЧӦ��ʹFe3++SCN![]() [Fe(SCN)]2+ƽ����ϵ�е�Fe3+��SCN��ϳ�[Fe(SCN)]2+�Ļ�����٣���Һ��ɫ��dz��
[Fe(SCN)]2+ƽ����ϵ�е�Fe3+��SCN��ϳ�[Fe(SCN)]2+�Ļ�����٣���Һ��ɫ��dz��
ԭ��ڣ�SCN������Fe2+��Ӧ������ɫ������ӣ���һ��ʹFe3++SCN![]() [Fe(SCN)]2+ƽ�����ƣ���ɫ���Ա�dz��
[Fe(SCN)]2+ƽ�����ƣ���ɫ���Ա�dz��
��֪��Mg2+��SCN����ϣ�����С�����������ʵ�飺

�ɴ��Ʋ⣬ʵ�������ɫ���Ա�dz����ԭ����___________________________��