��Ŀ����
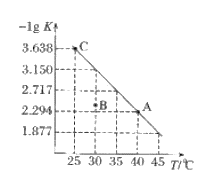
����Ŀ��ijƷ������Ƭ��Ѫ������ȥ���º��Ե�����ɫ����Ҫ�ɷ������������������������Ļ����ij�о���ѧϰС��Ϊ�ⶨ�ò�Ѫ�������������ĺ�����������ͼ1̽����
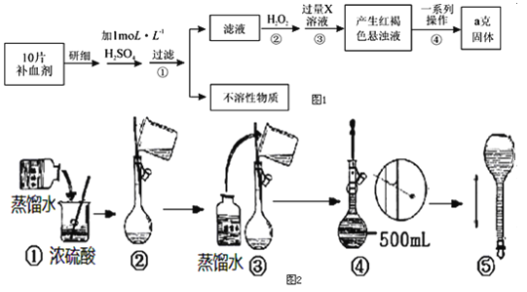
��ش��������⣺
��ʵ��ʱ��10molL-1��Ũ��������100mL1molL-1H2SO4��Һ��
��1��������Ͳ��ȡ____mL����Ũ����������ơ�
��2�������ʵ�������У��ٽ�ͷ�ιܡ����ձ�������Ͳ���ܲ�����������ϡ����ʱ����ȱ�ٵIJ���������____�����������߱��Ĺ�����____������ţ���
A������һ�����ȷŨ�ȵ���Һ
B������������Һ
C��������������Ͳ��ȡһ�������Һ��
D�������ܽ��������
��3����ͼ2���ƹ���ʾ��ͼ�У�������У�����ţ�_____��
��4�����в������������Ƶ�ϡ��������ʵ���Ũ��ƫ�͵���______����ĸ����
A������Ͳ��ȡŨ����ʱ���Ӱ�Һ��
B����Ũ����ϡ�ͺ�δ��ȴ�����±㽫��Һת��������ƿ
C������ƿ������ˮϴ��δ����
D������ʱ����Һ��
E��δϴ���ձ��Ͳ�����
F��ת�Ƶ�����ƿ�Ĺ����У�����������Һ����
G������Ͳ��ȡŨ���ᵹ��С�ձ���������ˮϴ����Ͳ����ϴ��Һת����С�ձ���
H���ò��������Ͳ��ȡŨ����
��1��������в����ĺ��ɫ�����ǣ��ѧʽ��_____��
��2���Ӻ��ɫ������Һ���õ��������ij�����һϵ�й���������Ļ�������Ϊ��
a��____��b��ϴ�ӣ�c�����գ�d����ȴ��
��3�����Ƶù��������Ϊag����ÿƬ��Ѫ���к���������������Ϊ____ g��
��4��д������ڵ����ӷ���ʽ��______��
���𰸡�10.0 100mL����ƿ A �٢� AEFH Fe(OH)3 ���� 0.19a 2Fe2++H2O2+2H+=2Fe3++2H2O
��������
������1��Ũ������ϡ��ǰ�����ʵ����ʵ������ֲ��䣬������Ũ�������ΪVmL����100mL��10-3��1mol/L=10mol/L��V��10��3L�����V=10.0mL���ʴ�Ϊ��10.0��
��2������һ�����ʵ���Ũ�ȵ���Һʱ����Ҫ��������������ƽ����Ͳ���ձ���������������ƿ����ͷ�ιܣ����ȱ�ٵ�������100mL����ƿ������ƿ��Ϊ�������������������桢��Ӧ���ܽ⣬Ҳ���ܲ��������������ݻ���Һ��������ʴ�Ϊ��100mL����ƿ��A��
��3��ϡ��Ũ����ʱ�ǽ�Ũ���������ڵ���ˮ�У����ò��������Ͻ��裬ʹ����������Ѹ����ɢ������ʱӦƽ�ӣ��������Ӻ��ӣ��ʴ�Ϊ���٢ܣ�
��4������![]() �������
�������
A������Ͳ��ȡŨ����ʱ���Ӱ�Һ�棬��ȡŨ����ƫ�٣�ϡ��������ʵ���Ũ��ƫ�ͣ���A�������⣻
B����Ũ����ϡ�ͺ�δ��ȴ�����±㽫��Һת��������ƿ�������º���Һ���ƫС��ϡ��������ʵ���Ũ��ƫ�ߣ���B���������⣻
C������ƿ������ˮϴ��δ�����ϡ��������ʵ���Ũ��û��Ӱ�죬��C���������⣻
D������ʱ����Һ�棬��Һ���ƫС��ϡ��������ʵ���Ũ��ƫ�ߣ���D�������⣻
E��δϴ���ձ��Ͳ�����������ƿ����������ʵ���ƫ�٣�ϡ��������ʵ���Ũ��ƫ�ͣ���E�������⣻
F��ת�Ƶ�����ƿ�Ĺ����У�����������Һ����������ƿ������ƫ�٣�ϡ��������ʵ���Ũ��ƫ�ͣ���F�������⣻
G������Ͳ��ȡŨ���ᵹ��С�ձ���������ˮϴ����Ͳ����ϴ��Һת����С�ձ��У���ȡŨ����ƫ�ߣ�ϡ��������ʵ���Ũ��ƫ�ߣ���G���������⣻
H���ò��������Ͳ��ȡŨ���ᣬŨ�������ƫ�٣�ϡ��������ʵ���Ũ��ƫ�ͣ���H���������⣻
�ʴ�ѡ��AEFH��
������1��������ǽ�Fe3+ת��Ϊ����������������Ӧ���ӷ���ʽΪFe3����3NH3��H2O=Fe(OH)3����3NH4������ú��ɫ����ΪFe(OH)3���ʴ�Ϊ��Fe(OH)3��
��2����������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ�ӡ����ա���ȴ��Ȼ��������������������ʴ�Ϊ�����ˣ�
��3��������ԭ���غ㣬ag����������Ԫ�ص�������Ϊ10Ƭ��Ѫ������������������ÿƬ��Ѫ������������������Ϊ�� =0.19ag���ʴ�Ϊ��0.19a��
=0.19ag���ʴ�Ϊ��0.19a��
��4���������������������ӱ�˫��ˮ�����������ӣ���Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O���ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�