��Ŀ����
 A��B��C��D��E�dz�����������ʣ�������ת����ϵ����ȥ��������Ʒ��
A��B��C��D��E�dz�����������ʣ�������ת����ϵ����ȥ��������Ʒ��
��1����DΪ������EΪNaOH��CΪNaAlO2������A������Ϊ______��Bת��ΪC�Ļ�ѧ����ʽ��______��
��2����AΪ��̬���ʣ�BΪƯ�۵���Ч�ɷݣ�C����Ư���ԣ���C�Ļ�ѧʽΪ______��Aת��ΪB�Ļ�ѧ����ʽ��______
��3����A��B��C��Ϊ���ε�ˮ��Һ��D��E��Ϊ±�ص��ʣ�д��Bת��ΪC�����ӷ���ʽ��______��
�⣺��1����DΪ������EΪNaOH��CΪNaAlO2����ת����ϵA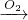 B
B C��֪��AΪAl��BΪAl2O3������ת����ϵ�����������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ����ʽΪ��Al2O3+2NaOH=2NaAlO2+H2O��
C��֪��AΪAl��BΪAl2O3������ת����ϵ�����������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ����ʽΪ��Al2O3+2NaOH=2NaAlO2+H2O��
�ʴ�Ϊ������Al2O3+2NaOH=2NaAlO2+H2O��
��2��AΪ��̬���ʣ�BΪƯ�۵���Ч�ɷݣ�BΪCa��ClO��2����A B��֪��AΪCl2��DΪCa��OH��2��C����Ư���ԣ���ת����ϵ��֪��CΪHClO���������������Ʒ�Ӧ�����Ȼ��ơ����������ˮ����Ӧ����ʽΪ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��
B��֪��AΪCl2��DΪCa��OH��2��C����Ư���ԣ���ת����ϵ��֪��CΪHClO���������������Ʒ�Ӧ�����Ȼ��ơ����������ˮ����Ӧ����ʽΪ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��
�ʴ�Ϊ��HClO��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��
��3����A��B��C��Ϊ���ε�ˮ��Һ��D��E��Ϊ±�ص��ʣ���ת����ϵ��֪������E��D��A�������ӣ�AΪNaI�ȣ�DΪBr2��BΪNaBr��EΪCl2��CΪNaCl������ת����ϵ��
�������廯�Ʒ�Ӧ�����Ȼ������嵥�ʣ���Ӧ���ӷ���ʽΪ��2Br-+Cl2=Br2+2Cl-���ʴ�Ϊ��2Br-+Cl2=Br2+2Cl-��
��������1����DΪ������EΪNaOH��CΪNaAlO2����ת����ϵA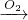 B
B C��֪��AΪAl��BΪAl2O3������ת����ϵ��
C��֪��AΪAl��BΪAl2O3������ת����ϵ��
��2��AΪ��̬���ʣ�BΪƯ�۵���Ч�ɷݣ�BΪCa��ClO��2����A B��֪��AΪCl2��DΪCa��OH��2��C����Ư���ԣ���ת����ϵ��֪��CΪHClO��
B��֪��AΪCl2��DΪCa��OH��2��C����Ư���ԣ���ת����ϵ��֪��CΪHClO��
��3����A��B��C��Ϊ���ε�ˮ��Һ��D��E��Ϊ±�ص��ʣ���ת����ϵ��֪������E��D��A�������ӣ�AΪNaI�ȣ�DΪBr2��BΪNaBr��EΪCl2��CΪNaCl������ת����ϵ��
����������������ת������ʽ����Al��Cl��Ԫ�ص��ʼ��仯����֮����ת����ϵ����ѧ�������д��ּ�ڿ���ѧ����Ԫ�ػ�����֪ʶ�������ճ̶ȣ�ע�⣨3���з�����������ϲ�ֹһ�֣�
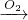 B
B C��֪��AΪAl��BΪAl2O3������ת����ϵ�����������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ����ʽΪ��Al2O3+2NaOH=2NaAlO2+H2O��
C��֪��AΪAl��BΪAl2O3������ת����ϵ�����������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ����ʽΪ��Al2O3+2NaOH=2NaAlO2+H2O���ʴ�Ϊ������Al2O3+2NaOH=2NaAlO2+H2O��
��2��AΪ��̬���ʣ�BΪƯ�۵���Ч�ɷݣ�BΪCa��ClO��2����A
 B��֪��AΪCl2��DΪCa��OH��2��C����Ư���ԣ���ת����ϵ��֪��CΪHClO���������������Ʒ�Ӧ�����Ȼ��ơ����������ˮ����Ӧ����ʽΪ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��
B��֪��AΪCl2��DΪCa��OH��2��C����Ư���ԣ���ת����ϵ��֪��CΪHClO���������������Ʒ�Ӧ�����Ȼ��ơ����������ˮ����Ӧ����ʽΪ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O���ʴ�Ϊ��HClO��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��
��3����A��B��C��Ϊ���ε�ˮ��Һ��D��E��Ϊ±�ص��ʣ���ת����ϵ��֪������E��D��A�������ӣ�AΪNaI�ȣ�DΪBr2��BΪNaBr��EΪCl2��CΪNaCl������ת����ϵ��
�������廯�Ʒ�Ӧ�����Ȼ������嵥�ʣ���Ӧ���ӷ���ʽΪ��2Br-+Cl2=Br2+2Cl-���ʴ�Ϊ��2Br-+Cl2=Br2+2Cl-��
��������1����DΪ������EΪNaOH��CΪNaAlO2����ת����ϵA
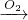 B
B C��֪��AΪAl��BΪAl2O3������ת����ϵ��
C��֪��AΪAl��BΪAl2O3������ת����ϵ����2��AΪ��̬���ʣ�BΪƯ�۵���Ч�ɷݣ�BΪCa��ClO��2����A
 B��֪��AΪCl2��DΪCa��OH��2��C����Ư���ԣ���ת����ϵ��֪��CΪHClO��
B��֪��AΪCl2��DΪCa��OH��2��C����Ư���ԣ���ת����ϵ��֪��CΪHClO����3����A��B��C��Ϊ���ε�ˮ��Һ��D��E��Ϊ±�ص��ʣ���ת����ϵ��֪������E��D��A�������ӣ�AΪNaI�ȣ�DΪBr2��BΪNaBr��EΪCl2��CΪNaCl������ת����ϵ��
����������������ת������ʽ����Al��Cl��Ԫ�ص��ʼ��仯����֮����ת����ϵ����ѧ�������д��ּ�ڿ���ѧ����Ԫ�ػ�����֪ʶ�������ճ̶ȣ�ע�⣨3���з�����������ϲ�ֹһ�֣�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��֪A��B��C��D��E�Ƕ�����ԭ�������������������Ԫ�أ�Aԭ����Ԫ�����ڱ���ԭ�Ӱ뾶��С��B��Eͬ���壬��E��ԭ��������B��������C��D�ǽ��������ǵ����������������ˮ������˵������ȷ���ǣ�������
| A�������ӵİ뾶��C��D��E��B | B����ҵ�ϳ��õ�ⷨ�Ƶ�C��D�ĵ��� | C���ȶ��ԣ�A2B��A2E | D������D������ұ��ijЩ���۽��� |

 2DB3����3.2gDB2��ȫת��ΪDB3����ʱ����akJ��1mol DB3������ȫת��Ϊ��ˮ�������bkJ��������33.6L DB2��ȫ�������������Ӧ����
2DB3����3.2gDB2��ȫת��ΪDB3����ʱ����akJ��1mol DB3������ȫת��Ϊ��ˮ�������bkJ��������33.6L DB2��ȫ�������������Ӧ����
