��Ŀ����
����Ŀ��ʵ�����г��ø��ϼ���ȡ���࣬�����Լ���RMgX�����Ʒ��ǣ�RX+Mg ![]() RMgX��RΪ������XΪ±�أ������Լ��ɷ�������ת�䣺
RMgX��RΪ������XΪ±�أ������Լ��ɷ�������ת�䣺 
��R��R���������ͬ��ͬ��������
��AΪԭ�Ϻϳ������춡���� ![]() �����������£����ַ�Ӧ�P��Ӧ����û���г�����A��Ҫ��Դ��ʯ���ѽ�����A�IJ�������Ϊ����ʯ�ͻ���ˮƽ�ı�־��
�����������£����ַ�Ӧ�P��Ӧ����û���г�����A��Ҫ��Դ��ʯ���ѽ�����A�IJ�������Ϊ����ʯ�ͻ���ˮƽ�ı�־��
�Իش�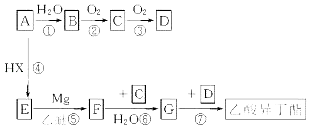
��1�����������У����ڻӳɷ�Ӧ���ǣ���д��ţ� ��
��2��д��F�Ľṹ��ʽ ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ����Ӧ�� �� ��Ӧ�� ��
���𰸡�
��1���٢ܢ�
��2��CH3CH2MgX
��3��CH2=CH2+H2O��CH3CH2OH�� CH3CH2CH��OH��CH3+CH3COOH ![]()
![]() +H2O
+H2O
���������⣺��1��������������֪�٢�Ϊ�ӳɷ�Ӧ�����ӳɷ�Ӧ���ʴ�Ϊ���٢ܢޣ���2��������������֪FΪCH3CH2MgX���ʴ�Ϊ��CH3CH2MgX����3����Ӧ������ϩ��ˮ�ļӳɷ�Ӧ����Ӧ��ѧ����ʽΪCH2=CH2+H2O��CH3CH2OH��
��Ӧ�ߵĻ�ѧ����ʽΪCH3CH2CH��OH��CH3+CH3COOH ![]()
![]() +H2O��
+H2O��
�ʴ�Ϊ��CH2=CH2+H2O��CH3CH2OH��CH3CH2CH��OH��CH3+CH3COOH ![]()
![]() +H2O��
+H2O��
�ɺϳ����̿�֪��AΪCH2=CH2����ˮ�����ӳɷ�Ӧ����BΪCH3CH2OH���Ҵ���������������CΪCH3CHO����ȩ��һ������������Ӧ����DΪCH3COOH��
��ϩ��HX�����ӳɷ�Ӧ����EΪCH3CH2X��E��Mg�����������·�����Ӧ����FΪCH3CH2MgX��F����ȩ�����ӳɷ�Ӧ����CH3CH2CH��OMgX��CH3���ٷ���ˮ��õ�GΪCH3CH2CH��OH��CH3��G�����ᷢ��������Ӧ�õ������춡�����Դ������

 �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�

