��Ŀ����
����������������Һ��Ӧ����Ӧ�����ӷ���ʽ��ȷ����
| A��0.2mol������300mL1mol/L������������Һ��� 2Al3����3SO42����3Ba2����6OH����2Al(OH) 3����3BaSO4�� |
| B��0.2mol������200mL1mol/L������������Һ��� Al3����SO42����Ba2����4OH����AlO2����BaSO4����2H2O |
| C��һ����������������ȫ��Ӧ�������ɳ������ʵ������� Al3����2SO42����2Ba2����4OH����AlO2����2BaSO4����2H2O |
| D��һ������������Һ�м�������������Һ�����ɳ������ʵ������ |
AC
�������������A����ȷ��B���������������㣬�����������������ᱵ������C�����ɳ������ʵ�������ʱ����������ȫ���ܽ�ʱ����ȷ��D���ɷ���ʽ��֪����������ʣ�࣬��������������Ӧ���ܹ��棬����
���㣺�������ӷ���ʽ�����ж��й����⡣

�����������ڷǵ���ʣ���ˮ��Һ�ܵ������(����)
| A��Cl2 | B��SO3 |
| C��HCl | D��Na2SO4 |
M��N����Һ�ֱ�������ʮ���������е����ֺ��������ӣ�K����Na����H+��NH4����Fe3���� A13+��Cl�D��OH�D��NO3�D��S2�D��CO32�D��SO42�D����֪����Һ�������Ӹ�����ͬ��M��Һ���������ֻ�����֣���N��Һ���������Ӧ����
| A��OH�D��CO32�D��SO42�D | B��S2�D��Cl�D��SO42�D |
| C��CO32�D��NO3�D��S2�D | D��Cl�D��SO42�D��NO3�D |
ijNa2CO3��NaAlO2�Ļ����Һ����μ���1 mol/L�����ᣬ�����Һ��CO32-��HCO3-��AlO2-��Al3�������ʵ��������������Һ������仯��ϵ��ͼ��ʾ��������˵����ȷ����
| A��ԭ�����Һ�е�CO32-��AlO2-�����ʵ���֮��Ϊ1��2 |
| B��V1��V2��l��5 |
| C��M��ʱ���ɵ�CO2Ϊ0��05 mol |
| D��a���߱�ʾ�����ӷ���ʽΪAlO2-��H����H2O==Al��OH��3�� |
�������ӷ���ʽ��д��ȷ����
| A����������NaOH��Һ����ͬŨ�ȵ�����Ca(HCO3)2��Һ��Ca2++2HCO3-+2OH-=CaCO3��+2H2O+CO32- |
| B����Ba(OH)2��Һ����μ���NH4HSO4��Һ���պó�����ȫBa2++OH-+H++ SO42-=BaSO4��+H2O |
C�����ð�˾ƥ�ֹ�������ˮ���ᣨ ����Ӧ���ɾ���ע��NaHCO3��Һ�� ����Ӧ���ɾ���ע��NaHCO3��Һ�� + 2 HCO3�� �� + 2 HCO3�� �� + 2 CO2�� + 2 H2O + 2 CO2�� + 2 H2O |
| D����FeI2��Һ�м�������ˮ 2Fe2++Cl2=2Fe3++2Cl- |
ijNa2CO3��NaAlO2�Ļ����Һ����μ���1mol��L-1�����ᣬ�����Һ�е�CO32-��HCO3-��AlO2-��Al3+�����ʵ��������������Һ������仯��ϵ��ͼ��ʾ��������˵������ȷ����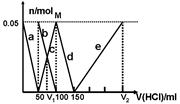
| A��M��ʱ���ɵ�CO2Ϊ0mol |
| B��ԭ�����Һ�е�CO32-��AlO2-�����ʵ���֮��Ϊ1��2 |
| C��V1��V2=1��4 |
| D��a���߱�ʾ�����ӷ���ʽΪ��AlO2- +H+ + H2O��Al(OH)3�� |
�������ӷ���ʽ��д��ȷ����
A��NaHCO3��Һ�е�ˮ�⣺HCO3����H2O H3O����CO32�� H3O����CO32�� |
| B���Ȼ�����Һ��ͨ�����⣺2Fe3����H2S��2Fe2����S����2H�� |
| C��CaCl2��Һ��ͨ��CO2: Ca2����CO2��H2O��CaCO3����2H�� |
| D��NaHSO4��Һ����μ���Ba(OH)2��Һ��ǡ�ó����ԣ� |
���и�����������Һ���ܴ����������( )��
| A��Na����Al3����Cl����SO42- | B��Cu2����Cl����NO3-��OH- |
| C��Ca2����Na����CO32-��NO3- | D��H����SO42-��NO3-��OH�� |