��Ŀ����
��1��������������Ĺ�����������Ӧ����������������� ��������CH3CH2OH��CH3CHO��Ӧѡ�� ������ĸ����A��NaOH��Һ B��������Һ C������
��������
 ��
�� Ӧѡ�� ������ĸ����
Ӧѡ�� ������ĸ����A��KMnO4��Һ B����ˮ C��Na2CO3��Һ
��������
 ��
�� Ӧѡ�� ������ĸ����
Ӧѡ�� ������ĸ����A��AgNO3��Һ B��NaOH��Һ C��FeCl3��Һ
��2�����л���ѧ�У�ͬ���칹���ձ�������� ����ʽΪC4H9Br���л��ﹲ�� �ֲ�ͬ�ṹ�����У�һ���л���ͨ����ȥ��Ӧ��ת��Ϊ2-��ϩ����д������ȥ��Ӧ�Ļ�ѧ����ʽ ����һ���л���ĺ˴Ź�������ͼ����ʾֻ��һ���壬��д�����л���Ľṹ��ʽ�� ��
��3���۶������Ҷ�������PES����һ������ɽ���ľ������������ϱ�Ĥ��ʳƷ��װ��������Ϸ������Ź㷺��Ӧ�ã���ṹ��ʽ���£�

�پ۶������Ҷ�������PES���������ֵ���ͨ�� ��Ӧ���Ӧ���ͣ��Ƶõģ��γɸþۺ�������ֵ����� �� ��
�������ֵ����֮��Ҳ�����γ�һ�ְ�Ԫ��״������д���û�״������Ľṹ��ʽ�� ��
���𰸡���������1�������л���Ĺ������ж����ʵ���ͬ����ѡ�������ע��ȩ�������ǻ������ʣ�
��2��C4H9Br��ͬ���칹����C4H9-��������CH3CH2CH2CH2Br��CH3CH2CHBrCH3����CH3��2CHCH2Br����CH3��3CBr4�֣������ĿҪ��ɽ����⣻
��3��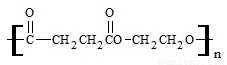 ��Ӧ�ĵ���ΪHOOCCH2CH2COOH��HOCH2CH2OH�����߿ɷ������۷�Ӧ��Ҳ�ɷ�����Ӧ���ɻ�����
��Ӧ�ĵ���ΪHOOCCH2CH2COOH��HOCH2CH2OH�����߿ɷ������۷�Ӧ��Ҳ�ɷ�����Ӧ���ɻ�����
����⣺��1����CH3CHO����-CHO������������Һ����������Ӧ�����Կ���������Һ����CH3CH2OH��CH3CHO��
�ʴ�Ϊ��B��
�ڼױ��������Ը��������Һ����������ԭ��Ӧ��ʹ���Ը��������Һ��ɫ���������ܣ��ʴ�Ϊ��A��
�۱��ӿ���FeCl3��Һ������Ӧ����Һ����ɫ��Ϊ������Ӧ���ʴ�Ϊ��C��
��2��C4H9Br��ͬ���칹����C4H9-��������CH3CH2CH2CH2Br��CH3CH2CHBrCH3����CH3��2CHCH2Br����CH3��3CBr4�֣�
����ͨ����ȥ��Ӧ��ת��Ϊ2-��ϩ��ΪCH3CH2CHBrCH3��
��Ӧ�ķ���ʽΪCH3CH2CHBrCH3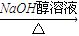 CH3CH=CHCH3+H2O����һ���л���ĺ˴Ź�������ͼ����ʾֻ��һ���壬˵��ֻ����1��H��ӦΪ��CH3��3CBr����дΪ
CH3CH=CHCH3+H2O����һ���л���ĺ˴Ź�������ͼ����ʾֻ��һ���壬˵��ֻ����1��H��ӦΪ��CH3��3CBr����дΪ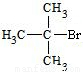 ���ʴ�Ϊ��
���ʴ�Ϊ��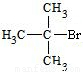 ��
��
�ʴ�Ϊ��CH3CH2CHBrCH3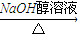 CH3CH=CHCH3+H2O��
CH3CH=CHCH3+H2O��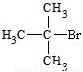 ��
��
��3��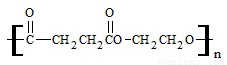 ��Ӧ�ĵ���ΪHOOCCH2CH2COOH��HOCH2CH2OH�����߿ɷ������۷�Ӧ��Ҳ�ɷ�����Ӧ���ɻ�����Ϊ
��Ӧ�ĵ���ΪHOOCCH2CH2COOH��HOCH2CH2OH�����߿ɷ������۷�Ӧ��Ҳ�ɷ�����Ӧ���ɻ�����Ϊ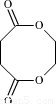 ��
��
�ʴ�Ϊ��HOOCCH2CH2COOH��HOCH2CH2OH��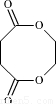 ��
��
���������⿼���л�����ƶ��Լ�������Ŀ�Ѷ��еȣ�ע������л���Ĺ����ŵĽṹ�����ʣ�Ϊ������Ĺؼ����״���Ϊͬ���칹����жϣ�ע���жϷ�����
��2��C4H9Br��ͬ���칹����C4H9-��������CH3CH2CH2CH2Br��CH3CH2CHBrCH3����CH3��2CHCH2Br����CH3��3CBr4�֣������ĿҪ��ɽ����⣻
��3��
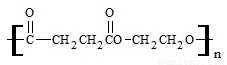 ��Ӧ�ĵ���ΪHOOCCH2CH2COOH��HOCH2CH2OH�����߿ɷ������۷�Ӧ��Ҳ�ɷ�����Ӧ���ɻ�����
��Ӧ�ĵ���ΪHOOCCH2CH2COOH��HOCH2CH2OH�����߿ɷ������۷�Ӧ��Ҳ�ɷ�����Ӧ���ɻ���������⣺��1����CH3CHO����-CHO������������Һ����������Ӧ�����Կ���������Һ����CH3CH2OH��CH3CHO��
�ʴ�Ϊ��B��
�ڼױ��������Ը��������Һ����������ԭ��Ӧ��ʹ���Ը��������Һ��ɫ���������ܣ��ʴ�Ϊ��A��
�۱��ӿ���FeCl3��Һ������Ӧ����Һ����ɫ��Ϊ������Ӧ���ʴ�Ϊ��C��
��2��C4H9Br��ͬ���칹����C4H9-��������CH3CH2CH2CH2Br��CH3CH2CHBrCH3����CH3��2CHCH2Br����CH3��3CBr4�֣�
����ͨ����ȥ��Ӧ��ת��Ϊ2-��ϩ��ΪCH3CH2CHBrCH3��
��Ӧ�ķ���ʽΪCH3CH2CHBrCH3
 CH3CH=CHCH3+H2O����һ���л���ĺ˴Ź�������ͼ����ʾֻ��һ���壬˵��ֻ����1��H��ӦΪ��CH3��3CBr����дΪ
CH3CH=CHCH3+H2O����һ���л���ĺ˴Ź�������ͼ����ʾֻ��һ���壬˵��ֻ����1��H��ӦΪ��CH3��3CBr����дΪ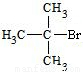 ���ʴ�Ϊ��
���ʴ�Ϊ��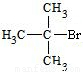 ��
���ʴ�Ϊ��CH3CH2CHBrCH3
 CH3CH=CHCH3+H2O��
CH3CH=CHCH3+H2O��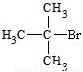 ��
����3��
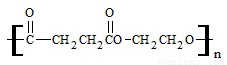 ��Ӧ�ĵ���ΪHOOCCH2CH2COOH��HOCH2CH2OH�����߿ɷ������۷�Ӧ��Ҳ�ɷ�����Ӧ���ɻ�����Ϊ
��Ӧ�ĵ���ΪHOOCCH2CH2COOH��HOCH2CH2OH�����߿ɷ������۷�Ӧ��Ҳ�ɷ�����Ӧ���ɻ�����Ϊ ��
���ʴ�Ϊ��HOOCCH2CH2COOH��HOCH2CH2OH��
 ��
�����������⿼���л�����ƶ��Լ�������Ŀ�Ѷ��еȣ�ע������л���Ĺ����ŵĽṹ�����ʣ�Ϊ������Ĺؼ����״���Ϊͬ���칹����жϣ�ע���жϷ�����

��ϰ��ϵ�д�
 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
�����Ŀ
 ��
�� Ӧѡ��
Ӧѡ�� ��
�� Ӧѡ��
Ӧѡ��


 ��
�� ��Ӧѡ��
��Ӧѡ��
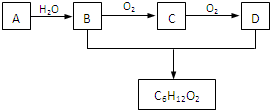

 ��1��������������Ĺ�����������Ӧ�����������������
��1��������������Ĺ�����������Ӧ����������������� ��
�� ��Ӧѡ��
��Ӧѡ��

 ��
�� ��Ӧѡ�� ___ (����ĸ)��
��Ӧѡ�� ___ (����ĸ)�� ����һ���л���ĺ˴Ź�������ͼ��1H�˴Ź�����ͼ������ʾһ���壬��д�����л���Ľṹ��ʽ���£� ��
����һ���л���ĺ˴Ź�������ͼ��1H�˴Ź�����ͼ������ʾһ���壬��д�����л���Ľṹ��ʽ���£� ��
 __________________��
__________________�� ��
�� ��Ӧѡ��
___ (����ĸ)��
��Ӧѡ��
___ (����ĸ)��