��Ŀ����
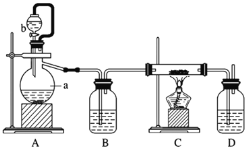
����Ŀ����ˮMgBr2������������ʵ���Ҳ���þм��Һ��Ϊԭ���Ʊ���ˮMgBr2,װ����ͼ��ʾ(�г�װ��ʡ��)��
��֪:��Mg��Br2���ҷ�Ӧ���ų���������; MgBr2 ����ǿ��ˮ��;
��MgBr2 + 3C2H5OC2H5![]() MgBr2��3C2H5OC2H5��
MgBr2��3C2H5OC2H5��

ʵ����Ҫ��������:
����1:������ƿ��װ��10 gþм(þ����ĥ���������)��
150 mL��ˮ����;����B�м���15 mLҺ�壬����װ��;��
����2:��ֹˮ�У�����ͨ�˸���ĵ�����ֱ������ȫ����������ƿ��;
����3:��Ӧ��Ϻ�ָ������£����˳�ȥþ����Һת������һ�������ƿ�У���ȴ��0��C������.���壬���˵������Ѻ��廯þ�ֲ�Ʒ;
����4:�ñ�ϴ�Ӵֲ�Ʒ����ѹ���ˣ��������Ѻ��廯þ�����������1609��C�ֽ����ˮMgBr2��
�ش���������:
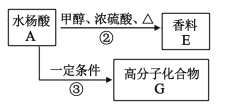
��1��MgBr2 ���γɹ��̿��õ���ʽ��ʾΪ_____________
��2������A��������_______;����B��������___________.ʵ��ǰ����A��B��������ƿ�ڱھ��豣�ָ��ԭ����__________________
��3��ʵ����,______________(������������������)�ø������������ﵪ��,������___________��
��4����ȥ����ˮԡ��������ƿ������MgBr2��ͬʱ�����ܻ�������������X, 1 mol X��50 mol e-,�仯ѧʽΪ______________________________________________��
��5������4���ü�ѹ����(����������ѹǿ��ʹ��Һ���ٷ���)������װ�ÿ�������ѹ���˵���________________________(�����)��

��6��Ϊ�ⶨ��Ʒ�Ĵ���(�ٶ����ʲ����뷴Ӧ)������EDTA (��дΪY4-����ɫ)����Һ�ζ��������TΪָʾ��(pH=6.3~11.6ʱ����ɫ,pH>11.6ʱ�Գ�ɫ)����֪: Mg2+�����T�γɵ������(Mg2+_���T)�ʾƺ�ɫ��Mg2+��Y4-�γɵ�MgY2-Ϊ��ɫ;��pHԼΪ9�Ļ�����Һ�еζ�����Ӧ�����ӷ���ʽ�ɼ�ʾΪ: Mg2+ + Y4-=MgY2-, Mg2+-��� T+Y4- =MgY2- +���T��
���жϵζ��յ������Ϊ__________________.
�ڲⶨǰ���ȳ�ȡ0.2500g��ˮMgBr2��Ʒ���ܽ����2�����T��Һ��ָʾ������0. 0500 mol��L-1 EDTA����Һ�ζ����յ㣬�ظ����εζ���ƽ������EDTA����Һ26. 60 mL,������ˮMgBr2��Ʒ�Ĵ�����___________________.
���𰸡�![]() �����������Ѻ������� ��ƿ MgBr2����ǿ��ˮ�ԣ���Ҫ����װ�ñ��ָ��� ���� þм��������Ӧ����MgO����Ĥ�������������谭Mg��Br2��Ӧ Mg3N2 bc ���μ����һ��EDTA����Һʱ����Һ�ɾƺ�ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ֲ��� 97.89%
�����������Ѻ������� ��ƿ MgBr2����ǿ��ˮ�ԣ���Ҫ����װ�ñ��ָ��� ���� þм��������Ӧ����MgO����Ĥ�������������谭Mg��Br2��Ӧ Mg3N2 bc ���μ����һ��EDTA����Һʱ����Һ�ɾƺ�ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ֲ��� 97.89%
��������
��ʵ��Ҫ�Ʊ���ˮ�廯þ��������Ŀ��Ϣ��֪þ�������ҷ�Ӧ�����ų��������ȣ�������װ��Ϊ�ܱ���ϵ������ͨ����岻��̫�죬�Է����ҷ�Ӧ������ը�����Ը���ĵ���Ҫ����ͨ�룬��Bװ���е���Ҫ�����̳����廯þ����ǿ��ˮ�ԣ���������װ��Ҫ������ˮ��������Ҫװ�ø���ܷ�ֹ�����е�ˮ�������룻��Ӧ�л�ų������ȣ�Ϊ�˼�����ԭ�ϻӷ���ɵ���ģ���Ҫ��������������������ҩƷ��
(1)�廯þ�γɹ�����þԭ��ʧȥ�������ӣ�������ԭ�ӷֱ�õ�һ�������γ������ӣ�Ȼ��������������þ���ӽ�������廯þ���õ���ʽ��ʾΪ��![]() ��
��
(2)װ��AΪ���������ܣ�����������ӷ������������ܿ��������������Ѻ�������������B�Ľṹ��֪��Ϊ��ƿ��������Ŀ��Ϣ��֪MgBr2����ǿ��ˮ�ԣ���Ҫ����װ�ñ��ָ��
(3)��ʵ��Ҫ��þм��Һ�巴Ӧ�����廯þ������װ���в���������þ��Ӧ�����壬�����������ø���Ŀ����������ĵ�����þм��������Ӧ����MgO����Ĥ�������������谭Mg��Br2��Ӧ��
(4)����Ԫ���غ��֪������ӦΪMgԪ�غ�NԪ���γɵ����ʣ��ٽ��1mol�����ʺ���50mol���ӿ�֪������ӦΪMg3N2��
(5)��ѹ���˹�������Ҫ����ƿ���γɸ�ѹ�����Կ���������ѹ���˵�װ��Ϊbc��
(6)�ٸ��ݵζ�ԭ��������ָʾ����Mg2+�����T�γɵ������(Mg2+-���T)����ʱ��ҺΪ�ʾƺ�ɫ���ζ������з�����ӦMg2+-��� T+Y4- =MgY2- +���T���ζ��յ�����pH=9����Һ��ʾ����T����ɫ�����յ�����Ϊ�����μ����һ��EDTA����Һʱ����Һ�ɾƺ�ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ֲ��䣻
�����ݷ���ʽMg2++Y4-�TMgY2-�������廯þ�����ʵ���=0.0500mol/L��0.02660L=0.00133mol�����廯þ������Ϊ0.00133mol��184g/mol=0.24472g���廯þ�IJ�Ʒ�Ĵ���=![]() ��100%=97.89%��
��100%=97.89%��

����Ŀ��(1)���ʹ�õ��ƽ���ȼ����N��H����Ԫ����ɣ���ԭ�Ӹ���N��H=1��2����ˮ��Һ�Լ��ԣ����������Nԭ�ӵ��ӻ���ʽΪ______________________��
(2)Ц��(N2O)������������������������ʳ�ᵼ������������ҡ�Ԥ��N2O�ĽṹʽΪ________________________��
(3)Ԫ�صĻ�̬��̬ԭ�ӵõ�һ�������γ���̬��1������ʱ���ų�������������һ��������(E)����1���������ٻ��һ�����ӵ������仯�����ڶ��������ܣ�����Ԫ�ػ����ӵĵ��������������±���ʾ��
Ԫ�� | C1 | Br | I | O | O- |
�������ܣ�kJ��mol�� | 349 | 343 | 295 | 141 | ��780 |
����˵����ȷ����___________��
A����������Խ��˵��Խ�ѵõ�����
B��һ����̬����̬��ԭ�ӵõ�һ�����ӳ�ΪO2-ʱ�ų�141kJ������
C����Ԫ�صĵڶ����������ǣ�780kJ��mol
D����̬����̬��ԭ�ӵõ��������ӳ�ΪO2-��Ҫ��������
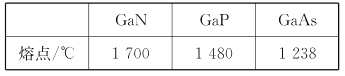
(4)�ڵ�����������м������ʯ(����A������)��������Al2O3�۵�����á�����ʯ������ԭ��Ϊ��2Al(OH)3+12HF+3Na2CO3=2A+3CO2��+9H2O�������������������գ�
�ٱ���ʯ�Ļ�ѧʽΪ____________________________��
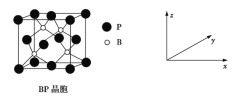
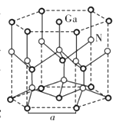
�ڱ���ʯ�����������ɣ�����ʯ�ľ����ṹ��ͼ����ʾ����λ�ڴ�������Ķ�������ģ���λ�ڴ��������12������е��8��С����������ģ���ô������������Ĵ�������������___________(��������)��
�۱���ʯ��Һ�в����ڵ�������������________________(��ѡ����ĸ)��
A ���Ӽ� B ���ۼ� C ��λ�� D ������ E ���»��� F ���
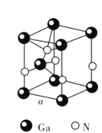
��Al���ʵľ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧��������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ��

����֪A1��ԭ�Ӱ뾶Ϊd cm��NA���������ӵ�������Al�����ԭ������ΪM������Alԭ�ӵ���λ��Ϊ________��Al������ܶ�Ϊ__________g��cm-3(����ĸ��ʾ)��
(5)�����Fe(CO)5���۵㣭20�棬�е�103�棬�������Ʊ�������Fe(CO)5�Ľṹ��ͼ��ʾ��

��Fe(CO)5������������__________���塣
�ڹ���Fe(CO)5������˵����ȷ����_____��
A��Fe(CO)5�ǷǼ��Է��ӣ�CO�Ǽ��Է���
B��Fe(CO)5��Feԭ����sp3�ӻ���ʽ��CO�ɼ�
C��1mol Fe(CO)5����10mol���
D����ӦFe(CO)5=Fe+5COû���»�ѧ������