��Ŀ����
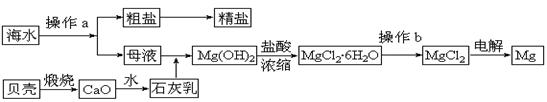
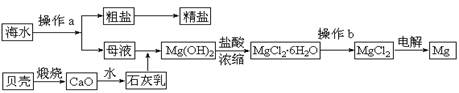
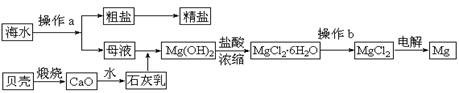
��12�֣�þ��һ����;�ܹ�Ľ������ϣ�Ŀǰ������60����þ�Ӻ�ˮ����ȡ����ˮ����Ҫ��NaCl��MgCl2�ȡ���Ҫ�������£�

(1)Ϊ��ʹMgCl2ת��ΪMg(OH)2���Լ��ٿ���ѡ��___________��ҪʹMgCl2��ȫת��Ϊ�����������Լ��ٵ���Ӧ___________����֤MgCl2����ȫת��ΪMg(OH)2�ķ�����_______________________________��
(2)�����Լ��٣��ܹ�����õ�Mg(OH)2�����ķ����� ____________________ ��
(3)�Լ��ڿ���ѡ��___________________________��
(4)��ˮMgCl2������״̬�£�ͨ�������Mg��д���÷�Ӧ�Ļ�ѧ����ʽ
���𰸡�

��4��MgCl2  Mg
+ Cl2��
Mg
+ Cl2��
��������

��ϰ��ϵ�д�
�����Ŀ