��Ŀ����
�о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����塣
(1)��CO2�뽹̿��������CO��CO�����������ȡ�
��֪��Fe2O3(s)��3C(s)=2Fe(s)��3CO(g)����H1����489.0 kJ��mol��1
C(s)��CO2(g)=2CO(g)����H2����172.5 kJ��mol��1��
��CO��ԭFe2O3���Ȼ�ѧ����ʽΪ________________________________
(2)ijʵ�齫CO2��H2����һ��������ܱ������У������ֲ�ͬ�����·�����Ӧ��CO2(g)��3H2(g) CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CH3OH�����ʵ�����ʱ��ı仯��ͼ��ʾ����ش��������⣺
CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CH3OH�����ʵ�����ʱ��ı仯��ͼ��ʾ����ش��������⣺

�ٸ÷�Ӧ��ƽ�ⳣ���ı���ʽΪK��________��
�����ߢ��Ӧ��ƽ�ⳣ����С��ϵΪK��________K��(����ڡ��������ڡ���С�ڡ�)��
������ͼa��b��c�����У�H2��ת�����ɸߵ��͵�˳����________(����ĸ)��

(3)�������������������£����������ѹ����ԭ����1/2����ԭƽ����ȣ������й�˵����ȷ����________(�����)��
a��������Ũ�ȼ�С
b������Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
c���״������ʵ�������
d������ƽ��ʱn(H2)/n(CH3OH)����
(1)��CO2�뽹̿��������CO��CO�����������ȡ�
��֪��Fe2O3(s)��3C(s)=2Fe(s)��3CO(g)����H1����489.0 kJ��mol��1
C(s)��CO2(g)=2CO(g)����H2����172.5 kJ��mol��1��
��CO��ԭFe2O3���Ȼ�ѧ����ʽΪ________________________________
(2)ijʵ�齫CO2��H2����һ��������ܱ������У������ֲ�ͬ�����·�����Ӧ��CO2(g)��3H2(g)
 CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CH3OH�����ʵ�����ʱ��ı仯��ͼ��ʾ����ش��������⣺
CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CH3OH�����ʵ�����ʱ��ı仯��ͼ��ʾ����ش��������⣺
�ٸ÷�Ӧ��ƽ�ⳣ���ı���ʽΪK��________��
�����ߢ��Ӧ��ƽ�ⳣ����С��ϵΪK��________K��(����ڡ��������ڡ���С�ڡ�)��
������ͼa��b��c�����У�H2��ת�����ɸߵ��͵�˳����________(����ĸ)��

(3)�������������������£����������ѹ����ԭ����1/2����ԭƽ����ȣ������й�˵����ȷ����________(�����)��
a��������Ũ�ȼ�С
b������Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
c���״������ʵ�������
d������ƽ��ʱn(H2)/n(CH3OH)����
(1)Fe2O3(s)��3CO(g)=2Fe(s)��3CO2(g)����H����28.5 kJ��mol��1
(2)��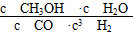 ���ڴ��ڡ���a��b��c
���ڴ��ڡ���a��b��c
(3)bc
(2)��
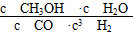 ���ڴ��ڡ���a��b��c
���ڴ��ڡ���a��b��c(3)bc
���ø�˹���ɼ���д����Ӧ���Ȼ�ѧ����ʽ���������ߢ��֪�������ߵ��¶Ȳ�ͬ�������ߢ���¶ȵ������ߢ���¶ȣ����ڸ÷�Ӧ�Ƿ��ȷ�Ӧ������K����K����H2����ʼͶ����Խ�٣���ƽ��ת����Խ�ߡ��������������������£����������ѹ����ԭ����1/2����ԭƽ����ȣ�������ֵ�Ũ�Ⱦ����������淴Ӧ���ʾ��ӿ죬ƽ�������ƶ�������������ʵ������ӣ���Ӧ���ת�����������´ﵽƽ��ʱn(H2)/n(CH3OH)��С��

��ϰ��ϵ�д�
�����Ŀ
 ������Ӧ�Ļ�ѧ��ӦΪ��__ ___
������Ӧ�Ļ�ѧ��ӦΪ��__ ___ CH3OCH3(g��+ 3H2O(g)
CH3OCH3(g��+ 3H2O(g)
 Fe3+(aq)+3OH-(aq) �� ��H=" a" kJ?mol-1
Fe3+(aq)+3OH-(aq) �� ��H=" a" kJ?mol-1 2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ,c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯����,��ͼ��ʾ��
2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ,c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯����,��ͼ��ʾ��


 N2(g)+CO2(g)+2H2O(g) ��H1="-867" kJ/mol
N2(g)+CO2(g)+2H2O(g) ��H1="-867" kJ/mol N2O4(g) ��H2="-56.9" kJ/mol
N2O4(g) ��H2="-56.9" kJ/mol


 TiCl4(l)��H="-804.2" kJ/mol
TiCl4(l)��H="-804.2" kJ/mol
 ��H
��H ��H1=��117.6kJ��mol��1
��H1=��117.6kJ��mol��1 CO (g)��H2O (g) ��H2=��41.2kJ��mol��1
CO (g)��H2O (g) ��H2=��41.2kJ��mol��1 ��
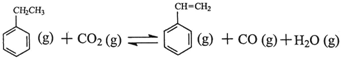
�� ����Ӧ�ﵽƽ��ʱ����ϩ��Ũ��Ϊ �� �����ú�
����Ӧ�ﵽƽ��ʱ����ϩ��Ũ��Ϊ �� �����ú�
 2NH3(g)���ص�,�ڸ���������ͼ2��,��������1 MPa��10 MPa������H2��ת�������¶ȱ仯����������ʾ��ͼ,�������������ߵ�ѹǿ��
2NH3(g)���ص�,�ڸ���������ͼ2��,��������1 MPa��10 MPa������H2��ת�������¶ȱ仯����������ʾ��ͼ,�������������ߵ�ѹǿ��
