��Ŀ����
T��ʱ����1L���ܱ������г���2mol CO2��6mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g��=CH3OH��g��+H2O��g����H=-49.0kJ/mol���H2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ������˵������ȷ���ǣ�������

| A��0��10min��v��H2��=0.3mol/��L?min�� | ||
B��T��ʱ��ƽ�ⳣ��K=
| ||
| C��T��ʱ��������Ӧ����64gCH3OH���ɣ�ͬʱ�ų�98.0kJ������ | ||
| D���ﵽƽ��������¶Ȼ��ٳ���CO2���壬���������H2��ת���� |

A.0��10min��H2�����ʵ�����6mol��СΪ3mol������v��H2��=
=0.3mol/��L?min������A��ȷ��
B��ƽ��ʱc��H2��=3mol/L��c��CO2��=1mol/L��c��CH3OH��=1mol/L��c��H2O��=1mol/L������ƽ�ⳣ��K=
=
��CO2��H2��ת���ʾ�Ϊ50%����B��ȷ��
C����ͼ��֪��ƽ��ʱ����1molCH3OH���ų�����Ϊ49kJ��64gCH3OH�����ʵ���Ϊ
=2mol�����Էų�98.0kJ����������C��ȷ��
D����H��0�������¶ȣ�ƽ�������ƶ���H2��ת���ʼ�С����ƽ����ٳ���CO2���壬ƽ�������ƶ����������H2��ת���ʣ���D����
��ѡD��
| ||
| 1L |
B��ƽ��ʱc��H2��=3mol/L��c��CO2��=1mol/L��c��CH3OH��=1mol/L��c��H2O��=1mol/L������ƽ�ⳣ��K=
| 1��1 |
| 1��33 |
| 1 |
| 27 |
C����ͼ��֪��ƽ��ʱ����1molCH3OH���ų�����Ϊ49kJ��64gCH3OH�����ʵ���Ϊ
| 64g |
| 32g/mol |
D����H��0�������¶ȣ�ƽ�������ƶ���H2��ת���ʼ�С����ƽ����ٳ���CO2���壬ƽ�������ƶ����������H2��ת���ʣ���D����
��ѡD��

��ϰ��ϵ�д�
�����Ŀ
 ��2010?������ģ�⣩T��ʱ����1L���ܱ������г���2mol CO2��6mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g��=CH3OH��g��+H2O��g����H=-49.0kJ/mol���H2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ������˵������ȷ���ǣ�������
��2010?������ģ�⣩T��ʱ����1L���ܱ������г���2mol CO2��6mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g��=CH3OH��g��+H2O��g����H=-49.0kJ/mol���H2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ������˵������ȷ���ǣ�������
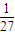 ��CO2��H2��ת�������
��CO2��H2��ת�������