��Ŀ����
ij������Һ�п��ܺ���K����Na����Mg2����Ba2����Al3����Fe3����Cl����I����CO![]() ��SO
��SO![]() �е�һ�ֻ��֡�ȡ����Һ��������ʵ�飺
�е�һ�ֻ��֡�ȡ����Һ��������ʵ�飺
����pH��ֽ���飬������Һ��ǿ���ԡ�
��ȡ��������Һ����������CCl4������������ˮ������CCl4����Ϻ�ɫ
����ȡ������Һ���������м���NaOH��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ�����������
����ȡ��������������Һ�������м���Na2CO3��Һ���а�ɫ�������ɡ�
��1��д���ڢڲ���Ӧ�����ӷ���ʽ___________________________���ɴ�Ҳ�����ų�______���ڣ�������________________________________�����ӷ���ʽ��ʾ��
��2����������ʵ����ʵȷ��������Һ�п϶����ڵ�������__________________
��3��������ȷ���Ƿ���ڵ�������_________________��
��1����1�֣�Cl2+2I-= I2+2Cl-����1�֣�___ Fe3+______��
��2�֣�2Fe3++2I-=2Fe2++I2
��2����2�֣�Ba2��, I-__��H+_______________________
��3����3�֣�__K����Na����Cl-_________________________

���ò�����պȡ����Һ����pH��ֽ�ϣ���ֽ�Ժ�ɫ��
����ȡ������Һ����BaCl2��Һ�����ɲ�����ϡ����İ�ɫ������
��ȡ�����ϲ���Һ �����ữ��������Һ��Ҳ���ɰ�ɫ������
���й��ڸ���Һ��˵������ȷ���ǣ�������
| A������Һ��һ��������Ba2+��HCO3- | B��ȡ������Һ����KSCN����Һ�Ժ�ɫ����ԭ��Һ��һ����Fe3+ | C������Һ��һ������SO42-��Cl- | D����ȡ����Һ���ٹ�NaOH��Һ���ȣ��Թܿڵ�ʪ���ɫʯ����ֽ����������ԭ ��Һ��һ��������NH4+ |
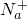 ��
��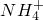 ��Fe2+��Fe3+��Ba2+��
��Fe2+��Fe3+��Ba2+��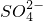 ��
��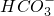 ��Cl-�������������ʵ�飺
��Cl-�������������ʵ�飺