��Ŀ����
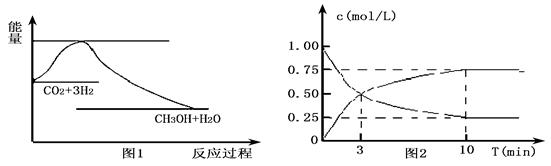
ʵ�֡����ܼ��š��͡���̼���á���һ����Ҫ���������ν�CO2ת��Ϊ�����õ���Դ��Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)����ͼ1��ʾ�÷�Ӧ����������(��λΪkJ��mol��1)�ı仯��
CH3OH(g)��H2O(g)����ͼ1��ʾ�÷�Ӧ����������(��λΪkJ��mol��1)�ı仯��

(1)���ڸ÷�Ӧ������˵���У���ȷ����________(����ĸ)��
A����H��0����S��0
B����H��0����S��0
C����H��0����S��0
D����H��0����S��0
(2)Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ1 L���ܱ������У�����1 mol��CO2��4 mol��H2��һ�������·�����Ӧ��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ2��ʾ��
CH3OH(g)��H2O(g)�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ2��ʾ��

�ٴӷ�Ӧ��ʼ��ƽ�⣬CH3OH��ƽ����Ӧ����v(CH3OH)��________��H2��ת����w(H2)![]() ________��
________��
�ڸ÷�Ӧ��ƽ�ⳣ������ʽK��________��
�����д�ʩ����ʹ��ѧƽ��������Ӧ�����ƶ�����________(����ĸ)��
A�������¶�
B����CH3OH(g)��ʱҺ�����
C��ѡ���Ч����
D���ٳ���1 mol��CO2��4 mol��H2
(3)25�棬1.01��105 Paʱ��16 gҺ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�363.3 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ��________��
(4)ѡ�ú��ʵĺϽ�Ϊ�缫�����������ơ��״���ˮ������Ϊԭ�ϣ������Ƴ�һ���Լ״�Ϊԭ�ϵ�ȼ�ϵ�أ��˵�صĸ���Ӧ�����ͨ���������________���������ĵ缫��Ӧʽ�ǣ�________��

 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�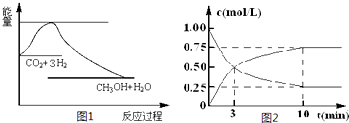

 CO2(g)+3H2(g)
CO2(g)+3H2(g) CH3OH(g)+H2O(g)����ͼ1��ʾ�÷�Ӧ�����������仯��
CH3OH(g)+H2O(g)����ͼ1��ʾ�÷�Ӧ�����������仯��