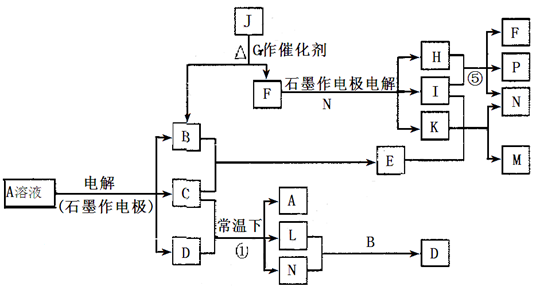
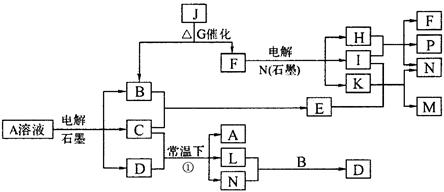
��Ŀ����
��֪AΪ��ɫ��Һ��B��C��I��KΪ���ʣ������Ϊ�����B��L��K ������Ϊ��ɫ��ζ���壬IΪ��ɫ�д̼�����ζ���塣GΪ��ɫ���壬F����ɫ��Ӧ����ɫ(���ܲ����۲�)����Ӧ���У�����F��P�����ʵ���֮��Ϊ1��1��������ת����ϵ����ͼ��www..com

�ش��������⣺
��1��P�ĵ���ʽ��_________��I����Ԫ����Ԫ�����ڱ��е�λ����________________��
��2��д����Ӧ�ٵ����ӷ���ʽ�� ______________________________________________��
��3��M��ˮ��Һ��___________������ԡ������ԡ������ԡ����������ӷ���ʽ˵��ԭ��______________________________________________________________
��4���ö��Ե缫���400.00mL A��Һ��һ��ʱ�������ҺpH��1����ʱ��Ҫ����Һ�м���___________��������Ϊ______g������ʹ��Һ�ָ������ǰ��״̬����������Һ����仯����
(14�֣���1��![]() ��2�֣����������ڢ�A�壻��2�֣�
��2�֣����������ڢ�A�壻��2�֣�
��2��3Cu + 8H+ + 2NO3�� = 3Cu2+ �� 2NO�� + 4H2O
��3�����ԣ�1�֣���Cu2+ + 2 H2O ![]() Cu(OH)2 + 2 H+
Cu(OH)2 + 2 H+
��4��CuO��������ͭ����2�֣���1.60 g��2�֣�������CuCO3��2.48 g��