��Ŀ����
�����������һ�ֳ����Ļ���ԭ�ϣ���SO2ͨ�밴һ��������ɵ�Na2S��Na2CO3�Ļ����Һ�У���ɵõ�Na2S2O3�����Ʊ���Ӧ����ʽΪ��2Na2S+Na2CO3+4SO2�T3Na2S2O3+CO2
��1�������ƻ����Һǰ�Ƚ�����ˮ�������һ��ʱ�����ã���Ŀ���� ��
��2���ø÷�����õ�Na2S2O3��H2O�����г�����һ���������ʣ�ij��ȤС�����������������ʳɷֽ���̽���������Ǹ���Ӧ�����������Ľᾧˮ����
[�������]
����1��������ֻ��Na2CO3����
����2��������ֻ��Na2S����
����3��
[��������]
��SO2+2H2S�T3S��+2H2O
��Na2S2O3�����ԡ�������Һ�н��ȶ�������������Һ����Ѹ�ٷ�Ӧ��
Na2S2O3+H2SO4�TNa2SO4+S��+SO2��+H2O
��CuSO4+H2S=CuS������ɫ��+H2SO4
[�ж���˼��]
ijͬѧȡ�����Ƶõľ�����������ϡH2SO4����������������ͨ��CuSO4��Һ�У�δ����ɫ�������ݴ���Ϊ����2������������Ϊ������Ƿ������ �������������������������˵�����ɣ�
[��Ʒ�������ʵ��]
���ڼ���1������±�ʵ�鷽���������ۣ�������ѡ����
��ѡʵ���Լ���3mol?L-1H2SO4��1mol?L-1NaOH������KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
��3����֪��2Na2S2O3+I2�T2NaI+Na2S4O6��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ����
��0.010mol?L-1�ĵ�ˮ���ж��ȡ���ζ������Na2S2O3?5H2O�ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ���� ��
��1�������ƻ����Һǰ�Ƚ�����ˮ�������һ��ʱ�����ã���Ŀ����
��2���ø÷�����õ�Na2S2O3��H2O�����г�����һ���������ʣ�ij��ȤС�����������������ʳɷֽ���̽���������Ǹ���Ӧ�����������Ľᾧˮ����
[�������]
����1��������ֻ��Na2CO3����
����2��������ֻ��Na2S����
����3��
[��������]
��SO2+2H2S�T3S��+2H2O
��Na2S2O3�����ԡ�������Һ�н��ȶ�������������Һ����Ѹ�ٷ�Ӧ��
Na2S2O3+H2SO4�TNa2SO4+S��+SO2��+H2O
��CuSO4+H2S=CuS������ɫ��+H2SO4
[�ж���˼��]
ijͬѧȡ�����Ƶõľ�����������ϡH2SO4����������������ͨ��CuSO4��Һ�У�δ����ɫ�������ݴ���Ϊ����2������������Ϊ������Ƿ������
[��Ʒ�������ʵ��]
���ڼ���1������±�ʵ�鷽���������ۣ�������ѡ����
��ѡʵ���Լ���3mol?L-1H2SO4��1mol?L-1NaOH������KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
| ʵ�鷽�� | ������ |
��0.010mol?L-1�ĵ�ˮ���ж��ȡ���ζ������Na2S2O3?5H2O�ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����
��������1��������ˮ�������һ��ʱ�����Ը��������ܽ����������ֹ��ʵ��������ţ�
��2�����ݼ���1�ͼ���2���������3��
[�ж���˼��]
����Ͷ�������֮����Է�����Ӧ���ɵ�����̼���ƿ��Ժ����ᷴӦ���ɶ�����̼�����ƿ��Ժ����ᷴӦ�������⣬������Ա��������������������̼����ʹ����ʯ��ˮ����ǣ��ݴ˼������ɷ֣�
��3������ʵ��ԭ�����ⵥ�ʵ���������Na2S2O3?5H2O�ĺ����dz���������ϵ�ģ�����Ӱ��ⵥ�ʺ�����������������
��2�����ݼ���1�ͼ���2���������3��
[�ж���˼��]
����Ͷ�������֮����Է�����Ӧ���ɵ�����̼���ƿ��Ժ����ᷴӦ���ɶ�����̼�����ƿ��Ժ����ᷴӦ�������⣬������Ա��������������������̼����ʹ����ʯ��ˮ����ǣ��ݴ˼������ɷ֣�
��3������ʵ��ԭ�����ⵥ�ʵ���������Na2S2O3?5H2O�ĺ����dz���������ϵ�ģ�����Ӱ��ⵥ�ʺ�����������������
����⣺��1��������ˮ�������һ��ʱ�䣬���Ը���ˮ�е��ܽ����������������ֹ���ʱ������������ʴ�Ϊ������ˮ�е��ܽ��������������
��2�����ݼ���1�ͼ���2���ó�����3�ǣ������к�Na2S��Na2CO3�������ʣ��ʴ�Ϊ�������к�Na2S��Na2CO3�������ʣ�
[�ж���˼��]
�����Na2S2O3��ϡH2SO4��Ӧ���ɵ�SO2��H2S������Ӧ��������H2S�ݳ��������ƶϲ�������̼���ƿ��Ժ����ᷴӦ���ɶ�����̼�����ƿ��Ժ����ᷴӦ�������⣬������Ա��������������������̼����ʹ����ʯ��ˮ����ǣ���������м������ᣬ���������岻��ʹƷ����ɫ����ʹ����ʯ��ˮ������ڣ�֤������ֻ��̼���ƣ�
�ʴ�Ϊ��
��3�����Na2S2O3?5H2O�ĺ���ԼΪ102%�������ĵĵ�ˮ����ƫ�ߣ������Ǿ����к������ʣ���Na2S�ȣ��ڵζ�ʱ���뷴Ӧ���岿��ʧȥ�ᾧˮ���ʴ�Ϊ�������к������ʣ���Na2S�ȣ��ڵζ�ʱ���뷴Ӧ���岿��ʧȥ�ᾧˮ��
��2�����ݼ���1�ͼ���2���ó�����3�ǣ������к�Na2S��Na2CO3�������ʣ��ʴ�Ϊ�������к�Na2S��Na2CO3�������ʣ�
[�ж���˼��]
�����Na2S2O3��ϡH2SO4��Ӧ���ɵ�SO2��H2S������Ӧ��������H2S�ݳ��������ƶϲ�������̼���ƿ��Ժ����ᷴӦ���ɶ�����̼�����ƿ��Ժ����ᷴӦ�������⣬������Ա��������������������̼����ʹ����ʯ��ˮ����ǣ���������м������ᣬ���������岻��ʹƷ����ɫ����ʹ����ʯ��ˮ������ڣ�֤������ֻ��̼���ƣ�
�ʴ�Ϊ��
| ʵ�鷽�� | ������ |
| ȡ������������ƿ�У���������3 mol?L-1 H2SO4�����ϴ������ܵ���Ƥ���������������嵼��������ͨ��ʢ������KMnO4��Һ��Ʒ����Һ������ʯ��ˮ��ϴ��ƿ�� | Ʒ����Һ����ɫ������ʯ��ˮ����ǣ������к�Na2CO3���� |
������������һ��̽�����ʵ���ɺͺ���֪ʶ���ۺ�ʵ���⣬����ѧ�������ͽ��������������Ѷȴ��ۺ���ǿ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


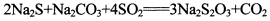 o
o
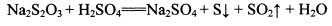
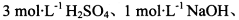 ����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
 ��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������
��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������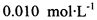 �ĵ�ˮ���ж��ȡ���ζ������
�ĵ�ˮ���ж��ȡ���ζ������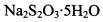 �ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��
�ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��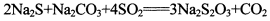 o
o
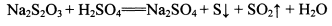
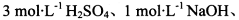 ����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
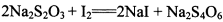 ��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������
��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������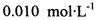 �ĵ�ˮ���ж��ȡ���ζ������
�ĵ�ˮ���ж��ȡ���ζ������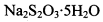 �ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��
�ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��