题目内容
已知:
Fe2O3(s)+3/2C(s)== 3/2CO2(g)+2Fe(s);△H=234.1KJ/mol
C(s)+ O2(g)== CO2(g); △H= -393.5KJ/mol
则4Fe(s)+ 3O2(g)== 2Fe2O3(s)的△H是
Fe2O3(s)+3/2C(s)== 3/2CO2(g)+2Fe(s);△H=234.1KJ/mol
C(s)+ O2(g)== CO2(g); △H= -393.5KJ/mol
则4Fe(s)+ 3O2(g)== 2Fe2O3(s)的△H是
[ ]
A.-1648.8KJ/mol
B.-824.4KJ/mol
C.+1648.8KJ/mol
D.-744.7KJ/mol
B.-824.4KJ/mol
C.+1648.8KJ/mol
D.-744.7KJ/mol
A

练习册系列答案
相关题目

 I.高炉炼铁是冶炼铁的主要方法,发生的主要反应为:
I.高炉炼铁是冶炼铁的主要方法,发生的主要反应为: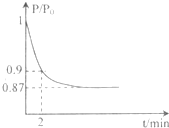 能源的开发利用与人类社会的可持续性发展息息相关.
能源的开发利用与人类社会的可持续性发展息息相关. 研究CO2的利用对促进低碳社会的构建具有重要的意义.
研究CO2的利用对促进低碳社会的构建具有重要的意义.