��Ŀ����
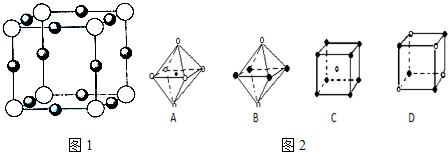
��Ԫ�ؿ��γɶ������ӣ���(1)1��![]() ���Ӻ���________�����ӣ�
���Ӻ���________�����ӣ�![]() �ĵ���ʽΪ________��
�ĵ���ʽΪ________��
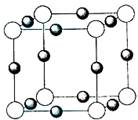
(2)�γ�![]() ���ӵ����Է��ӵĽṹʽ��________��
���ӵ����Է��ӵĽṹʽ��________��
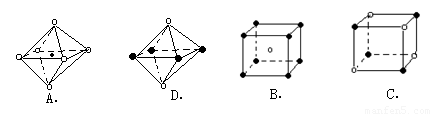
(3)д��![]() ������ǿ����Һ�з�Ӧ�����ӷ���ʽ________________________��
������ǿ����Һ�з�Ӧ�����ӷ���ʽ________________________��
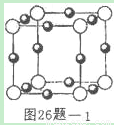
(4)����A�Ļ�ѧʽΪNH5����������ԭ������㶼����ϡ������ԭ�ӵ��������ӽṹ���������ʵ����Ⱦͷֽ���������壬�Իش�
�ٹ���A����________���壬���ĵ���ʽ________��
��A����ˮ����Һ��________��(��ᡱ������С�)����ԭ���û�ѧ����ʽ��ʾΪ________________________��
��A���ȷֽ�Ļ�ѧ����ʽ��________________________��
�𰸣�
������
������
| (1)22, (2) (3)N2H62- +2OH-====N2H4+2H2O (4)������, �ڼ�,NH4H+H2O====NH3��H2O+H2������NH5 
��ϰ��ϵ�д�
 ����ѧ��Ӯ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����Ŀ
|
 ��
��