��Ŀ����
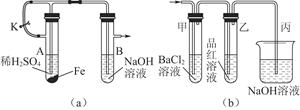
��ͼʵ��װ��������֤ijЩ���ʵ����ʡ����Թ�A��װ�������Ĺ���NaHCO3,DΪ�̶������ӲֽƬ���Իش��������⣺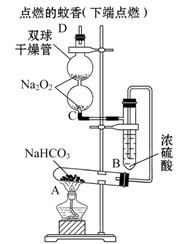
��1����ʵ���ʵ��Ŀ����__________________________________________��
��2����A�Թ��ڷ�����Ӧ�Ļ�ѧ����ʽ��_______________________________��
��3��Bװ�õ�������_________________________________________________��
��4����˫�������ڷ�����Ӧ�Ļ�ѧ����ʽΪ____________________________��
��5��ʵ��ʱ�۲쵽��ʵ��������_______________________________________��
����ʵ������˵��___________________________________________________��
��6������������ڵ�Na2O2����Na2O����ʵ��ʱ�۲쵽��ʵ��������___________��
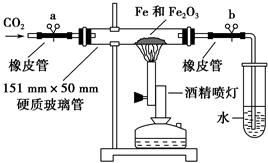
��1����֤CO2��Na2O2�ķ�Ӧ
��2��2NaHCO3 Na2CO3+H2O��+CO2��
Na2CO3+H2O��+CO2��
��3�����������е�ˮ����������CO2��
��4��2Na2O2+2CO2=2Na2CO3+O2
��5������ɫ��Na2O2��ת��Ϊ��ɫ��ĩ����ȼ������ȼ�ո��Ӿ��� Na2O2����CO2��Ӧ�����ɰ�ɫ��ĩ״���ʺ�O2
��6����ȼ��������Ϩ��
����

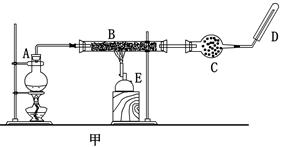
ʵ��Ŀ�ģ�̽������������ˮ��Ӧ�����Һ�μӷ�̪��Һ�ȱ�����ɫ��ԭ��
[���������]
��1�����ݹ���������ˮ��Ӧ��ԭ����2Na2O2 + 2H2O =" 4NaOH" + O2�������������ƹ�����ȫ�ܽⷴӦ�����Һ�еμӷ�̪��Ӧֻ�����������ɫ����ʵ���з��ַ�̪��������ɫ���ɴ�������µIJ��룺
A��������Ư����
B������������Ư����
C��
[ʵ�����ж�] ��������б���
| ʵ���� | 1 | 2 | 3 |
| ʵ��װ�� | 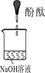 | 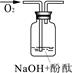 | 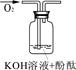 |
| ��֤���� | | C | |
| ʵ������ | ��Һ������ɫ | ||
| ʵ��˵�� | 1��2��ʵ����NaOH��Һ���� ����������ƹ��塱���������ƹ��塱�����������ƹ��塱������ˮ���Ƶġ� | ||
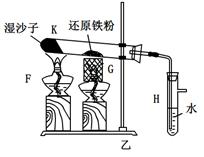
��2����������ʵ��������֣�����������ˮ��Ӧ�����У���Ԫ���γ����ȶ��Ļ������Һ�л�������һ�ֲ����ȶ�������Ư���Ե�����X��X�Ļ�ѧʽ�� ��
��3��������ͼװ�ö���Һ�в����ȶ������ʽ���̽�����ڢٴ�װ��������� ����ѡ���ͬ�����ڴ�װ��������� ��
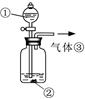
A����̪�Լ� B������������ˮ��Ӧ�����Һ
C���������� D���������ƹ������Ƶ���Һ
��4��������� ������������ˮ��Ӧ�Ļ�ѧ����ʽû��д��X����ԭ���� ��



 BaCO3��+H2O)
BaCO3��+H2O)