��Ŀ����
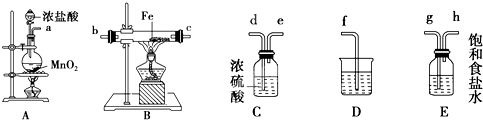
ij����С�齫��ͼ��ʾװ�ð�һ��˳�����ӣ���ʵ��������ȡһ������FeCl3����ͨ�����������ַ�Ӧ����

��ش��������⣺
(1)װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ________________��
(2)��װ�õ���ȷ����˳��Ϊ����дװ�ô��ţ�A�� _____��____��____��D��
(3)װ��C��������________________��д��װ��D�з�Ӧ�����ӷ���ʽ��____________________��
(4)��Ӧ��ʼ��װ��B��Ӳ�ʲ������ڵ�����Ϊ____________________�����Լ������������Ƿ���
Fe3+���Լ���________��
(5)��С��������ͼ��ʾװ���ռ�β������������������������
(1)װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ________________��
(2)��װ�õ���ȷ����˳��Ϊ����дװ�ô��ţ�A�� _____��____��____��D��
(3)װ��C��������________________��д��װ��D�з�Ӧ�����ӷ���ʽ��____________________��
(4)��Ӧ��ʼ��װ��B��Ӳ�ʲ������ڵ�����Ϊ____________________�����Լ������������Ƿ���
Fe3+���Լ���________��
(5)��С��������ͼ��ʾװ���ռ�β������������������������

������ͼ��ʾ����װ���ɸ���ܡ��齺�ܺ�50 mL�ζ��ܸ������װ���ɣ��˴����õζ�����______ �����ʽ����ʽ�����ζ��ܡ�
��Ϊ����߲�����ȷ�ԣ���ͼװ���е�Һ�����___________________���ռ����������ǰӦ���еIJ�����____________________��
�������ʼ����ʱ������ȷ��������ʱ�����ұߵζ���Һ�棬�ᵼ��������������______ ���ƫ����ƫС������Ӱ�족����
��Ϊ����߲�����ȷ�ԣ���ͼװ���е�Һ�����___________________���ռ����������ǰӦ���еIJ�����____________________��
�������ʼ����ʱ������ȷ��������ʱ�����ұߵζ���Һ�棬�ᵼ��������������______ ���ƫ����ƫС������Ӱ�족����
(1)4HCl(Ũ)+MnO2 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
(2)E��C��B
(3)����Cl2����ֹFeCl3��ˮ�⣻Cl2+2OH-=Cl-+ClO-+H2O
(4)�����أ��죩ɫ���̣�KSCN��Һ
(5)�ټ�ʽ���ڱ���NaCl��Һ�������ƶ��ζ��ܣ�ʹ��������Һ����ƽ����ƫ��
 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O(2)E��C��B
(3)����Cl2����ֹFeCl3��ˮ�⣻Cl2+2OH-=Cl-+ClO-+H2O
(4)�����أ��죩ɫ���̣�KSCN��Һ
(5)�ټ�ʽ���ڱ���NaCl��Һ�������ƶ��ζ��ܣ�ʹ��������Һ����ƽ����ƫ��

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ


 _________________________________��
_________________________________�� _________________________��
_________________________�� ��
��
 ͼ��ʾ����װ���ɸ���ܡ��齺�ܺ�50 mL�ζ��ܸ������װ���ɣ��˴����õζ�����________�����ʽ����ʽ�����ζ��ܡ�
ͼ��ʾ����װ���ɸ���ܡ��齺�ܺ�50 mL�ζ��ܸ������װ���ɣ��˴����õζ�����________�����ʽ����ʽ�����ζ��ܡ� �ᵼ��������������__________���ƫ����ƫС������Ӱ�족����
�ᵼ��������������__________���ƫ����ƫС������Ӱ�족����
