��Ŀ����
����˵����ȷ���ǣ� ��
A��þ�뼫ϡ���ᷴӦ��������淋����ӷ���ʽΪ:4Mg+6H++N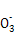 =4Mg2++N =4Mg2++N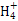 +3H2O +3H2O |
| B��������,0.1 mol/LһԪ��HA��Һ��c(OH-)/c(H+)=1��10-8,�����Һ��pH=3 |
| C����ͬ������,Ũ�Ⱦ�Ϊ0.01 mol/L��NH4Cl��Һ��NaCl��Һ��,ǰ�ߵ���������Ũ�ȴ��ں��ߵ���������Ũ�� |
| D�����ʵ���Ũ����ȵĴ��������������Һ�������Ϻ����Һ��:c(Na+)+c(H+)=c(CH3COO-)+c(OH-)+c(CH3COOH) |
B
A���ɡ�ԭ�Ӳ��غ�,ӦΪ4Mg+10H++N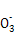 =4Mg2++N
=4Mg2++N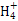 +3H2O,����;c(OH-)/c(H+)=1��10-8��c(OH-)��c(H+)=1��10-14����,�ɵ�c(H+)=1��10-3 mol��L-1,��pH=3,B��;������Һ������,NH4Cl��Һ�д���c(N
+3H2O,����;c(OH-)/c(H+)=1��10-8��c(OH-)��c(H+)=1��10-14����,�ɵ�c(H+)=1��10-3 mol��L-1,��pH=3,B��;������Һ������,NH4Cl��Һ�д���c(N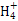 )+c(H+)=c(Cl-)+c(OH-),NaCl��Һ�д���c(Na+)+c(H+)=c(Cl-)+c(OH-),����NH4Cl��Һ�����Զ�NaCl��Һ������,��ǰ�ߵ�c(OH-)С�ں���,��c(Cl-)��Ϊ0.01 mol/L,��c(N
)+c(H+)=c(Cl-)+c(OH-),NaCl��Һ�д���c(Na+)+c(H+)=c(Cl-)+c(OH-),����NH4Cl��Һ�����Զ�NaCl��Һ������,��ǰ�ߵ�c(OH-)С�ں���,��c(Cl-)��Ϊ0.01 mol/L,��c(N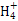 )+c(H+)��c(Na+)+c(H+),C��;������Һ������,CH3COONa��Һ�д���c(Na+)+c(H+)=c(CH3COO-)+c(OH-),D����
)+c(H+)��c(Na+)+c(H+),C��;������Һ������,CH3COONa��Һ�д���c(Na+)+c(H+)=c(CH3COO-)+c(OH-),D����
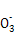 =4Mg2++N
=4Mg2++N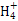 +3H2O,����;c(OH-)/c(H+)=1��10-8��c(OH-)��c(H+)=1��10-14����,�ɵ�c(H+)=1��10-3 mol��L-1,��pH=3,B��;������Һ������,NH4Cl��Һ�д���c(N
+3H2O,����;c(OH-)/c(H+)=1��10-8��c(OH-)��c(H+)=1��10-14����,�ɵ�c(H+)=1��10-3 mol��L-1,��pH=3,B��;������Һ������,NH4Cl��Һ�д���c(N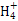 )+c(H+)=c(Cl-)+c(OH-),NaCl��Һ�д���c(Na+)+c(H+)=c(Cl-)+c(OH-),����NH4Cl��Һ�����Զ�NaCl��Һ������,��ǰ�ߵ�c(OH-)С�ں���,��c(Cl-)��Ϊ0.01 mol/L,��c(N
)+c(H+)=c(Cl-)+c(OH-),NaCl��Һ�д���c(Na+)+c(H+)=c(Cl-)+c(OH-),����NH4Cl��Һ�����Զ�NaCl��Һ������,��ǰ�ߵ�c(OH-)С�ں���,��c(Cl-)��Ϊ0.01 mol/L,��c(N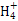 )+c(H+)��c(Na+)+c(H+),C��;������Һ������,CH3COONa��Һ�д���c(Na+)+c(H+)=c(CH3COO-)+c(OH-),D����
)+c(H+)��c(Na+)+c(H+),C��;������Һ������,CH3COONa��Һ�д���c(Na+)+c(H+)=c(CH3COO-)+c(OH-),D����
��ϰ��ϵ�д�
 С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д� ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
�����Ŀ
 2H2SO4
2H2SO4 +2H2O
+2H2O H2CO3+2OH¯
H2CO3+2OH¯ +H2O
+H2O
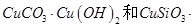
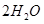 ��ͬʱ����
��ͬʱ����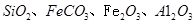 �����ʡ��Թ��ȸʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��
�����ʡ��Թ��ȸʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��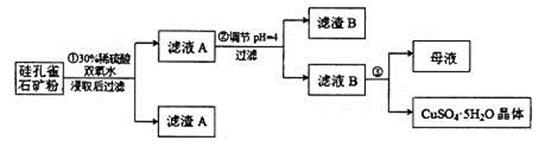
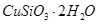 ������Ӧ�Ļ�ѧ����ʽ
������Ӧ�Ļ�ѧ����ʽ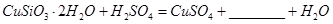 ��
��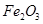
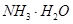
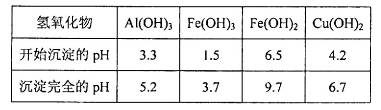
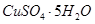 ���塣ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�
���塣ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�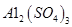 ������Һ��
������Һ��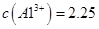 mol
mol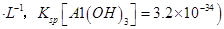 ______________��
______________�� 2OHһ+H2��+Cl2��
2OHһ+H2��+Cl2�� H2O��NH3��
H2O��NH3��


 ʮH2O
ʮH2O