��Ŀ����
���ú�ˮ��ȡ�壨Br2�������̿ɱ�ʾ���£�
��Br-�ĺ�ˮ��Br-�ĺ�ˮ��Br2�ĺ�ˮ����Ũ�����ữ��ͨCl2��ͨ������ˮ������Br2����H2SO4HBr��Һ�����ҺSO2ˮ��Һ��ͨCl2��CCl4��ˮ��ҺBr2/CCl4
��1����ɢ��з�Ӧ�Ļ�ѧ����ʽ
��2��������7����
��3���٢ڢۢܢݲ�����Ŀ����
a����ȡ���� b����ȡ���� c���ᴿ������ d�������壮
���������ú�ˮ��ȡ�壺��Br-�ĺ�ˮͨ������������Ũ�����ữ�ڣ������ۼ���������ͨCl2���������������ԣ����������������ɵ����壻�õ����嵥�ʵĺ�ˮ��������ͨ������ˮ����������Br2������ͨ����������SO2ˮ��Һ����Br2��ʹ������ת��Ϊ�����ᣬ����ΪH2SO4��HBr��Һ�����Һ���ﵽ�������Ŀ�ģ���ͨCl2�ޣ��������������ɵ����壬�����л��ܼ�CCl4����ȡ���ⵥ�ʣ��õ�ˮ��Һ��Br2/CCl4������������Ӻ�����л��ܼ�����Һ�з�������ʵ�ͻ����л��ܼ��������û��ܵ�����Һ��ķе㲻ͬ�����룬��ʵ�����Ϊ����
��1��ͨ����������SO2ˮ��Һ����Br2������������嵥�ʷ���������ԭ��Ӧ��BrԪ�صĻ��ϼ۽��͡�SԪ�صĻ��ϼ����ߣ�Br2����������
��2�������л��ܼ�CCl4����ȡ���ⵥ�ʣ�
��3������������Ũ�������������ữ�������ۼ���������ͨCl2���������������ɵ����壬������ͨ������ˮ����������Br2������ͨ������������Br2���ﵽ�������Ŀ�ģ�
��1��ͨ����������SO2ˮ��Һ����Br2������������嵥�ʷ���������ԭ��Ӧ��BrԪ�صĻ��ϼ۽��͡�SԪ�صĻ��ϼ����ߣ�Br2����������
��2�������л��ܼ�CCl4����ȡ���ⵥ�ʣ�
��3������������Ũ�������������ữ�������ۼ���������ͨCl2���������������ɵ����壬������ͨ������ˮ����������Br2������ͨ������������Br2���ﵽ�������Ŀ�ģ�
����⣺���ú�ˮ��ȡ�壺��Br-�ĺ�ˮͨ������������Ũ�����ữ�ڣ������ۼ���������ͨCl2���������������ԣ����������������ɵ����壻�õ����嵥�ʵĺ�ˮ��������ͨ������ˮ����������Br2������ͨ����������SO2ˮ��Һ����Br2��ʹ������ת��Ϊ�����ᣬ����ΪH2SO4��HBr��Һ�����Һ���ﵽ�������Ŀ�ģ���ͨCl2�ޣ��������������ɵ����壬�����л��ܼ�CCl4����ȡ���ⵥ�ʣ��õ�ˮ��Һ��Br2/CCl4��
��1��ͨ����������SO2ˮ��Һ����Br2����Ԫ�ػ��ϼ���0�۱�Ϊ-1�ۣ���������������Ԫ�ػ��ϼ���+4�۱�Ϊ+6�ۣ����������ǻ�ԭ����������Br2+SO2+2H2O�T2HBr+H2SO4��
�ʴ�Ϊ��Br2+SO2+2H2O�T2HBr+H2SO4��
��2���������Ȼ�̼�е��ܽ�ȴ�����ˮ�е��ܽ�ȣ����Ȼ�̼��ˮ�����ܣ������Ȼ�̼�͵ⲻ��Ӧ���ʿ������Ȼ�̼ͨ��������7����ȡ��ˮ�еĵⵥ�ʣ��õ�Br2/CCl4��
�ʴ�Ϊ�������л��ܼ�CCl4��ȡ���ⵥ�ʣ�
��3������������Ũ�������������ữ�������ۼ���������ͨCl2���������������ɵ����壬���ӷ���������ͨ������ˮ����������Br2������ͨ������������Br2���ﵽ�������Ŀ�ģ�
�ʴ�Ϊ��d��
��1��ͨ����������SO2ˮ��Һ����Br2����Ԫ�ػ��ϼ���0�۱�Ϊ-1�ۣ���������������Ԫ�ػ��ϼ���+4�۱�Ϊ+6�ۣ����������ǻ�ԭ����������Br2+SO2+2H2O�T2HBr+H2SO4��
�ʴ�Ϊ��Br2+SO2+2H2O�T2HBr+H2SO4��
��2���������Ȼ�̼�е��ܽ�ȴ�����ˮ�е��ܽ�ȣ����Ȼ�̼��ˮ�����ܣ������Ȼ�̼�͵ⲻ��Ӧ���ʿ������Ȼ�̼ͨ��������7����ȡ��ˮ�еĵⵥ�ʣ��õ�Br2/CCl4��
�ʴ�Ϊ�������л��ܼ�CCl4��ȡ���ⵥ�ʣ�
��3������������Ũ�������������ữ�������ۼ���������ͨCl2���������������ɵ����壬���ӷ���������ͨ������ˮ����������Br2������ͨ������������Br2���ﵽ�������Ŀ�ģ�
�ʴ�Ϊ��d��
���������⿼�������ú�ˮ��ȡ��ʵ�鷽����ƣ��漰���ʵķ��롢���顢������ѡȡ��֪ʶ�㣬�������ʵ��ص㼰����ѡȡ��Ӧ�ķ���ͼ��鷽������������������ѡȡ������ע�����ʵ�����̺�ʵ��ԭ����ע�����ʵ�������Ҫ���ע�������ȡ����ѡȡ����֪ʶ�㶼�dz�����㣬��Ҳ���״��㣮��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 С�����ϵ�д�
С�����ϵ�д�
�����Ŀ
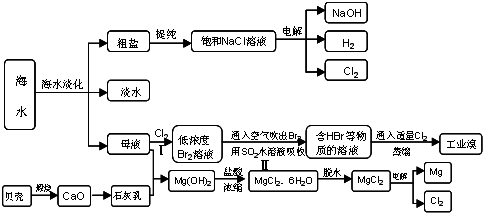


 MgCl2 + 6H2O���÷�ӦҪ��HCl�����н��У�ԭ���� ��
MgCl2 + 6H2O���÷�ӦҪ��HCl�����н��У�ԭ���� ��