��Ŀ����
(10��)������Ԫ��A��B��C��D�У�0.5mol AԪ�ص����ӵõ�6.02��1023�����ӱ���ԭΪ����ԭ�ӣ�0.4g A��������ǡ����100ml 0.2mol/L��������ȫ��Ӧ��Aԭ�Ӻ���������Ŀ��������Ŀ��ȣ�BԪ��ԭ�Ӻ�������������Ŀ�ȵ�һ���1����C����AԪ�ص����Ӷ�1�����Ӳ㣬DԪ�ص�ԭ�Ӻ���ڶ���ȵ�һ���2�����ӡ��ش��������⣺
��1��C�D�Ľṹʾ��ͼΪ ��
��Cͬ�����һ����Ԫ�ص���̬�⻯����ȶ��Ա�HC��________���ǿ������������
��Cͬ�����һ����Ԫ�ص���̬�⻯��ķе��HC��________����ߡ��͡�����
��2��Ԫ��D�����������ĵ���ʽΪ_______��D����̬�⻯��Ķ��ȴ�����_____�ֽṹ��
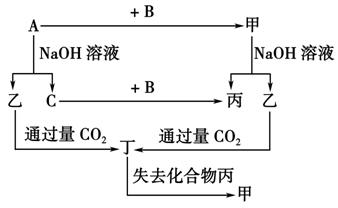
��3����ҵ��ұ������A�Ļ�ѧ����ʽΪ_____________________________________��
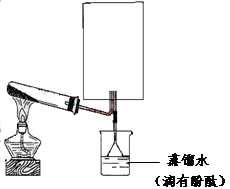
��4����������A��B�õ������Ӳ��뵽����������Һ�п������ԭ��أ�������������_______���û�ѧʽ��д���������缫��Ӧ��___________________________________��
��1��  �� ǿ�� �ߣ� ��2��
�� ǿ�� �ߣ� ��2�� �� 1�� ��ÿ��1�֣�
�� 1�� ��ÿ��1�֣�
��3��MgCl2(����) Mg+ Cl2����2�֣�
Mg+ Cl2����2�֣�
��4��Mg ��1�֣��� Al-3e- + 4OH- = AlO2- + 2H2O��2�֣�
����

 ��У����ϵ�д�
��У����ϵ�д�

 ___________________________
___________________________