��Ŀ����
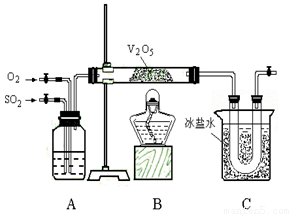
��11�֣�ij�о���ѧϰС���ͬѧΪ��ʵ�������ֶ�������������Ĺ��̣�����ȡ�������������壬�������ͼ��ʾ��װ�á���֪����������ˮ�������Ტ�ų������ȣ������γ���������ҵ�ϳ���Ũ����������������
�Իش�
��1����֪6.4 g ��������������������̬�������ų�9.83 kJ��������Ӧ���Ȼ�ѧ����ʽΪ____________________________________________________��[��Դ:ѧ+��+��]
��2��Aװ�ÿ������۲��������������Ľ�������ʵ��ʱʹA����������ð�����������������ð�������������������Ŀ����___________________________��
��3�������װ�õ��������Ҽ���ҩƷ��ʼ����ʵ�顣��ʱ����Ӧ�ý��еIJ�����________________________________��
��4��Cװ�������ռ�������������a gͭ������Ũ���ᷴӦ�ƶ�������ʵ�����ʱ�õ�b g ��������ʵ���ж��������ת���ʲ�С��_________________��
��5��Ϊ�˼���ʵ��Ի�������Ⱦ���������β������װ�ã����װ��ͼ��
__________________________________________________________ ��
��11�֣�
��1��SO2��g��+ O2��g��
O2��g�� SO3��g������H=-98.3kJ/mol ��3�֣�
SO3��g������H=-98.3kJ/mol ��3�֣�
��2����߶��������ת���ʣ� ��2�֣�
��3�����������ȣ���2�֣�
��4�� ��
�� ��100%��2�֣�
��100%��2�֣�
��5����β��ͨ��ʢ��Ũ�����ϴ��ƿ����ͨ��ʢ������������Һ��ϴ��ƿ����ͨ��ʢ��������ʯ�ҵĸ���ܵȣ���2�֣�
��������

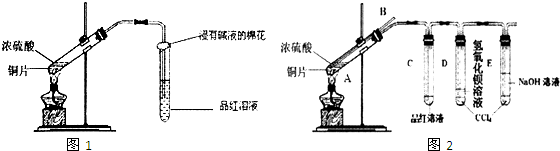
��1��д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
��2����С��ͬѧ��ʵ���з�������ʵ��װ�����൱���֮������ʵ�鲻����ȫ������ɻ�����Ⱦ�ȣ�Ϊ�Ľ�ʵ�������˽�SO2�����ʣ�����ͬѧ�ʵ����ۺ�����ʦ�Ľ������������ͼ2ʵ��װ�ã�
�����������B������
�ڶ��Թ�A�е�ŨH2SO4��ͭƬ���м��ȣ�����E�Թ����������ݳ���Ʒ����Һ�ܿ���ɫ��������δ��D�Թ�������������Һ���ֻ��ǣ�Ϊ̽��D�Թ���δ���ֻ��ǵ�ԭ��С��ͬѧ�ڻ�ѧ�ֲ���ֻ���ĵ��������ʳ����µ��ܽ�����ݣ�
| ���� | �ܽ�ȣ�g/100ˮ�� | ���� | �ܽ�ȣ�g/100ˮ�� |
| Ca��OH��2 | 0.173 | Ba��OH��2 | 3.89 |
| CaCO3 | 0.0013 | BaSO3 | 0.016 |
| Ca��HCO3��2 | 16.60 |
��Ϊ��֤D�Թ�����Һ����ɣ�����������ʵ�飬����������������ʵ�����ݣ�
| ʵ�鷽�� | ���� |
| 1��ȡ������Һ���Թ��У�����ϡ���ᣬ���ȣ� ��ʪ�����ɫʯ����ֽ�������ɵ����壮 |
|
| 2��ȡ������Һ���Թ��У����� |
 ������Ƥ�����н�ǿ����ʴ�ԣ��������г��õĽ�������֮һ����п��������Ƥ�ı����㣬���Ĥ�ĺ�ȼ����ȶ�Ҳ�����ж϶Ʋ���������Ҫָ�꣮ij�о���ѧϰС��Ϊ�˲ⶨ��п��Ƥ�ĺ�ȣ�����������ʵ�鷽����
������Ƥ�����н�ǿ����ʴ�ԣ��������г��õĽ�������֮һ����п��������Ƥ�ı����㣬���Ĥ�ĺ�ȼ����ȶ�Ҳ�����ж϶Ʋ���������Ҫָ�꣮ij�о���ѧϰС��Ϊ�˲ⶨ��п��Ƥ�ĺ�ȣ�����������ʵ�鷽���� Al��OH��3+3H+��Cu2++2H2O
Al��OH��3+3H+��Cu2++2H2O Cu��OH��2+2H+��2Al+6H+=2Al3++3H2��
Cu��OH��2+2H+��2Al+6H+=2Al3++3H2��