��Ŀ����
����Ŀ��A��B��C��D��E��FΪǰ������ԭ�������������������Ԫ�أ�A��C��Dԭ�Ӿ�������δ�ɶԵ��ӣ�A��B��Cͬ���ڣ�A��D��B��F�ֱ�ͬ���壬E���������������Ľ�������ش��������⣺
��1��E�Ƚ��ȶ������Ӻ�������Ų�ʽ_____________________________��
��2��A��B��C�ĵ�һ��������С�����˳��Ϊ_________________![]() ��Ԫ�ط��ű�ʾ
��Ԫ�ط��ű�ʾ![]() ��B��C�ļ��⻯�����������������ʵĻ�ѧʽ___________��
��B��C�ļ��⻯�����������������ʵĻ�ѧʽ___________��
��3��C��D�γɵ����ʵľ���������____________��IT��ҵ�иþ�����������____________��
��4����A��B��C����Ԫ���е�һ�ֻ�����Ԫ���γɵķ����У��еĻ�Ϊ�ȵ����壬д������һ��ȵ�����Ļ�ѧʽ��______![]() ��д����Ӧ�Ľṹʽ_______________��
��д����Ӧ�Ľṹʽ_______________��
��5��B�ĵ��ʾ�����![]() �����ƣ���һ�������к�B��ԭ�Ӹ���Ϊ____
�����ƣ���һ�������к�B��ԭ�Ӹ���Ϊ____![]() �����γɵķ��ӿռ乹����_______��
�����γɵķ��ӿռ乹����_______��
��6������˪����һ�ֺ�C��F�Ļ��������ӽṹ��ͼ1��ʾ���û�����ķ���ʽΪF4C6��Fԭ�Ӳ�ȡ______�ӻ���C��D��E��ɵĻ�����ľ�����ͼ2���侧������Ϊa pm�������ܶ�Ϊ___________________g/cm3���г�ʽ�Ӽ��ɣ�����٤������ΪNAmol-1����

���𰸡� ![]() ��
��![]()
![]()
![]() ԭ�Ӿ��� ���ά
ԭ�Ӿ��� ���ά ![]() ��CO
��CO ![]() ��
��![]() 8 ������ sp3
8 ������ sp3 ![]()
��������A��B��C��D��E��FΪǰ������ԭ�������������������Ԫ�أ�A��C��Dԭ�Ӿ�������δ�ɶԵ��ӣ���A��������Ϊ6��C��C��������Ϊ8��O��DΪ������Ϊ14��Si����A��B��Cͬ���ڣ���B��N��A��D��B��F�ֱ�ͬ���壬��F��As��E���������������Ľ�������EΪFe������������AΪC��BΪN��CΪO��DΪSi��EΪFe��FΪAs��
��1��Fe�Ƚ��ȶ���������Fe3+,���������Ų�ʽΪ![]() ��
��![]() ��
��
��2��ͬ����Ԫ����ԭ������������һ�����ܳ��������ƣ���N��2p�ܼ�����3�����ӣ�Ϊ�����״̬�������ϵͣ����һ�����ܸ���O���ʵ�һ��������С�����˳��Ϊ��![]() ��
��![]() ��B��C�ļ��⻯�����������������ʾ��Ƿе���͵ģ�����H2O��NH3�о�����������е�ϸߣ�������������������ΪCH4��
��B��C�ļ��⻯�����������������ʾ��Ƿе���͵ģ�����H2O��NH3�о�����������е�ϸߣ�������������������ΪCH4��
��3��C��D�γɵ������Ƕ������裬����ԭ�Ӿ�����IT��ҵ�иþ��������������ά��
��4����C��N��O����Ԫ���е�һ�ֻ�����Ԫ���γɵķ����л�Ϊ�ȵ�������![]() ��CO����Ӧ�Ľṹʽ
��CO����Ӧ�Ľṹʽ![]() ��
��![]() ��
��
��5��N2�ľ�����![]() �����ƣ����Ծ����е������ӵĸ���Ϊ��8
�����ƣ����Ծ����е������ӵĸ���Ϊ��8![]() +6
+6![]() =4����һ�������к�N��ԭ�Ӹ���Ϊ2
=4����һ�������к�N��ԭ�Ӹ���Ϊ2![]() As�����γɵķ���ΪAsH3,��ռ乹��������NH3,Ϊ�����Ρ�
As�����γɵķ���ΪAsH3,��ռ乹��������NH3,Ϊ�����Ρ�
��6����ԭ�����γ�2�����ۼ���As�γ�3�����ۼ�����ͼ��֪������Oԭ����Ϊ6��Asԭ����Ϊ4�������仯ѧʽΪAs4O6��As�������5�����ӣ��γ�3�����ۼ���![]() �����Ӷ���Ϊ3�������1���µ��Ӷԣ�����As���ӻ���ʽΪsp3���ڸþ����ṹ�У�����O��Si��Fe�����к�Feԭ����ĿΪ8
�����Ӷ���Ϊ3�������1���µ��Ӷԣ�����As���ӻ���ʽΪsp3���ڸþ����ṹ�У�����O��Si��Fe�����к�Feԭ����ĿΪ8![]() =2����Si
=2����Si![]() +6
+6![]() =4����ѧʽΪFe2SiO4����Ħ������Ϊ204g/mol���������ܶ�Ϊ
=4����ѧʽΪFe2SiO4����Ħ������Ϊ204g/mol���������ܶ�Ϊ![]() =
=![]() =
=![]() g
g![]() cm-3,�ʴ�Ϊ��
cm-3,�ʴ�Ϊ��![]() ��
��

����Ŀ����25 ��ʱ���ܱ�������X��Y��Z��������ij�ʼŨ�Ⱥ�ƽ��Ũ�����±���
���� | X | Y | Z |
��ʼŨ��/mol��L��1 | 0.1 | 0.2 | 0 |
ƽ��Ũ��/mol��L��1 | 0.05 | 0.05 | 0.1 |
����˵����ȷ����
A. ��Ӧ�ɱ�ʾΪ��X(g)��Y(g) ![]() 2Z(g)
2Z(g)
B. ��Ӧ�ﵽƽ��ʱ��X��ת����Ϊ60%
C. �ڴ������£��÷�Ӧ��ƽ�ⳣ��ΪK=1600
D. ������������ʱ������ѹǿƽ�ⷢ���ƶ����ٴ���ƽ�⣬ƽ�ⳣ������
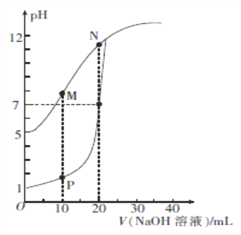
����Ŀ�������£�ijͬѧ�������백ˮ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
ʵ���� | ��ˮŨ��/mol��L-1 | ����Ũ��/mol��L-1 | �����ҺpH |
�� | 0.1 | 0.1 | pH��5 |
�� | c | 0.2 | pH��7 |
�� | 0.2 | 0.1 | pH��7 |
��ش�����������
��1���������û����Һc(OH-)��______ mol��L-1��
��2��������c______0.2������>����<��������������
��3���������û����Һ��������Ũ���ɴ�С��˳����_______��
��4���١������ð�ˮ�е� ����_______��������>����<��������������
����_______��������>����<��������������