��Ŀ����
������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ�ճ�ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ�����Ʒ���������ͼ��ʾװ�ã���Ӧԭ��Ϊ��CH3COOH��C2H5OH CH3COOC2H5��H2O
CH3COOC2H5��H2O
�����Ҫ��ش��������⣺
(1)ʵ��ʱ�����Ʒ�Ӧ���Һ�IJ����ǣ�________��
(2)����������ת���ʣ��ɲ�ȡ�Ĵ�ʩ�У�________��________�ȣ�
(3)������ͼ��ʾ��װ�����Ʊ�������������������������ƫ�ͣ���ԭ�����Ϊ��________��________�ȣ�

(4)�˷�Ӧ��Ũ����Ϊ���������ܻ����________��________�����⣮
(5)ʵ���б���Na2CO3��Һ��������(����ĸ���)________��
A���к�������Ҵ�
B���к����Ტ���ղ����Ҵ�
C�����������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�е�С�������ڷֲ�����
D�������������ɣ���������
(6)����ƿ���ռ���һ�����IJ����ֹͣ���ȣ���ȥ��ƿ��������Ȼ����һ������ɹ۲쵽������Ϊ________��
������
|
����(1)������ƿ�м����Ҵ���Ȼ��������ļ���Ũ��������ᣮ ����(2)�����Ҵ���Ũ�ȡ���ȥ������ ����(3)ԭ����������Ӧ�ͱ��������¶ȹ��ߣ������˸���Ӧ������Ч�����ã����ֲ���ӷ���(��������) ����(4)BC ����(5)��ƿ�е�Һ����������㣬�ϲ�����ɫ�ľ���ˮ����ζ����״Һ�壬�²�Һ���dz��ɫ |

 ��ҵ����ϵ�д�
��ҵ����ϵ�д�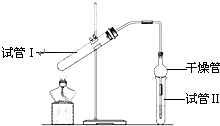 ������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ��������ͼ��װ���Ʊ�����������
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ��������ͼ��װ���Ʊ�������������1����̲IJ��õ�ʵ��װ�ò�ͬ����װ���в��������θ���ܣ��������ǣ�
��2��Ϊ��֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý���������4��ʵ�飮ʵ�鿪ʼ���þƾ���
��3min���ټ���ʹ֮����3min��ʵ�����������С�Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܢ����Լ� | �Թܢ��� �Լ� |
���� ���/cm |
| A | 2mL�Ҵ���1mL���ᡢ 1mL18mol?L-1 Ũ���� |
����Na2CO3 ��Һ |
3.0 |
| B | 2mL�Ҵ���1mL���� | 0.1 | |
| C | 2mL�Ҵ���1mL���ᡢ 3mL 2mol?L-1H2SO4 |
0.6 | |
| D | 2mL�Ҵ���1mL���ᡢ���� | 0.6 |
�ڷ���ʵ��
��3������������90g���Ҵ�138g����������Ӧ�õ�80g�����������Լ���÷�Ӧ�IJ���Ϊ
 ������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ����������ͼ��װ���Ʊ�����������
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ����������ͼ��װ���Ʊ����������� ������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ�ճ�ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��CH3COOH+C2H5OH
������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ�ճ�ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��CH3COOH+C2H5OH 


 ������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ�ճ�ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ���Һ�ҵ�Ʒ����ñ����ᡢ��ˮ�Ҵ���Ũ�����ϣ��ڼ��������·�Ӧ�Ƶã�
������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ�ճ�ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ���Һ�ҵ�Ʒ����ñ����ᡢ��ˮ�Ҵ���Ũ�����ϣ��ڼ��������·�Ӧ�Ƶã�