��Ŀ����
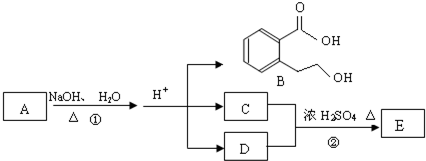
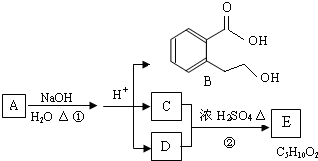
A��B��C��D��E��Ϊ������ˮ�Ĺ��壬���ǵ����ӿ����У�Na+��Ca2+��Ba2+��C1O-��HSO4-��CO32-��C1-��
�ֱ�ȡ���ǵ���Һ����ʵ�飬��Ҫ�������������£�
����A����Һ��ͨ�������̼���ټ���Ʒ����Һ����ɫ��ȥ��
�ڽ�B��C����Һ��ϣ����ɰ�ɫ�������ó���������E��Һ��
�۽�B��D����Һ��ϣ����ɰ�ɫ�������������������E��Һ�������ݲ�����ͬʱ���а�ɫ�������ڣ�
��A��E��Һ����ɫ��Ӧ���ʻ�ɫ��
��1��д���������ʵĻ�ѧʽ��A______��B______��C______��E______��
��2��д��E��Һ����������Ӧ��ʵ������Ϊ______��
��3����B����Һ�У�������������ϡ��E��Һ������Ҫ�����ʳɷ�Ϊ______�����ѧʽ��
��4��D�ڿ����г��ھ��û�ʧЧ���仯ѧ����ʽΪ______��
�⣺��1���������⣬��������ʹƷ����Һ��ɫ��ȥ�������Ǿ���ǿ�����Ե�HClO�����Ƶ�A�к���C1O-����ӦΪ��
2C1O-+CO2+H2O�TCO32-+2HClO��HClO��������ǿ������Ʒ���������ȶ���ɫ���ʶ���ɫ��
�������н�B��C����Һ��ϣ����ɰ�ɫ������������֪��Ϣ����ɫ����ΪCaCO3��BaCO3�����ó���������E��Һ��˵��E��ҺΪ������Һ����E��Һ��HSO4-�������ɫ����ΪCaCO3����ӦΪCaCO3+2H+�TCa2++CO2��+H2O�������ɫ����ΪBaCO3����ӦΪBaCO3+2H++SO42-�TBaSO4��+CO2��+H2O�����ó���������E��Һ����˸ó���ΪCaCO3��
�������н�B��D����Һ��ϣ����ɰ�ɫ�������������������E��Һ�������ݲ�����ͬʱ���а�ɫ�������ڣ���ϵ�������пɵó�B��D����Һ������ɰ�ɫ����ΪBaCO3�����������E��Һ��ɫ����ת��ΪBaSO4����B�к�CO32-��D�к�Ba2+��C�к�Ca2+������CaCO3��BaCO3��������ˮ�����BΪNa2CO3��Ca2+����C1O-��C1-�����CΪCaC12��Ca��C1O��2��
��������A��E��Һ����ɫ��Ӧ���ʻ�ɫ��˵��A��E����Na+����AΪNaClO��EΪNaHSO4��
�ʴ�Ϊ��NaC1O��Na2CO3��CaC12��Ca��C1O��2��NaHSO4��
��2��EΪNaHSO4����ˮ��Һ�е��룺NaHSO4�TNa++H++SO42-��H+������������Ӧ��6H++2Al�T2Al3++H2�����ʹ۲쵽����Ϊ�����������
�ʴ�Ϊ�������������
��3��B��ҺΪNa2CO3��Һ��E��ҺΪNaHSO4��Һ����B����Һ�л�����������ϡ��E��Һ��������Ӧ��
Na2CO3+NaHSO4�TNaHCO3+Na2SO4������Ҫ�����ʳɷ�ΪNaHCO3��Na2SO4��
�ʴ�Ϊ��Na2SO4��NaHCO3��
��4��D�к�Ba2+���ڿ����г��ھ��û�ʧЧ��˵��D���ȶ�����ȷ��DΪBa��ClO��2���Ϳ����еĶ�����̼��ˮ������Ӧ�������Ȼ����ʹ����ᣬ�������ټ���ֽ��ʧЧ������ʽΪ��Ba��ClO��2+CO2+H2O�TBaCl2+2HClO��2HClO 2HCl+O2����
2HCl+O2����
�ʴ�Ϊ��Ba��ClO��2+CO2+H2O�TBaCl2+2HClO��2HClO 2HCl+O2����
2HCl+O2����
��������1�����ݲ�����Ʒ����Һ��ɫ��ȥ���Ƶ�A������������ӣ���ϵ�����ܼ����Ƶ�A�����ݲ����ڡ��۶����ɰ�ɫ��������ȷ��B��̼������ӣ����B����ˮ��ȷ����B�����ɰ�ɫ����������E��Һ���Ƶ�E������������ӣ��������а�ɫ����������E��Һ���ɵ�C�������ӣ����������ɰ�ɫ�������������������E��Һ�������ݲ�����ͬʱ���а�ɫ�������ڣ���ȷ��D�������ӣ�����A��B��C��D��E��Ϊ������ˮ�Ĺ��壬������ӹ��漴���жϳ������ʣ�
��2��EΪNaHSO4��Һ�������H+������H+���������ķ�Ӧ�ó�����
��3��BΪNa2CO3��EΪNaHSO4�����ݷ����ķ�Ӧ��д�������T�ɣ�
��4��D�к�Ba2+���ڿ����г��ھ��û�ʧЧ�����뵽Ư�۵�ʧЧԭ������֪D��ClO-���ο�Ư�۵�ʧЧԭ��д������ʽ��
���������⽫���ӷ�Ӧ��Ԫ�ؼ��仯����֪ʶ�ں���һ�飬��Ҫ��ǿ���ۺ�˼ά������ͨ��ȫ�������ץסϸ�ڣ�������룬��ѵ��˼ά���а�����
2C1O-+CO2+H2O�TCO32-+2HClO��HClO��������ǿ������Ʒ���������ȶ���ɫ���ʶ���ɫ��
�������н�B��C����Һ��ϣ����ɰ�ɫ������������֪��Ϣ����ɫ����ΪCaCO3��BaCO3�����ó���������E��Һ��˵��E��ҺΪ������Һ����E��Һ��HSO4-�������ɫ����ΪCaCO3����ӦΪCaCO3+2H+�TCa2++CO2��+H2O�������ɫ����ΪBaCO3����ӦΪBaCO3+2H++SO42-�TBaSO4��+CO2��+H2O�����ó���������E��Һ����˸ó���ΪCaCO3��
�������н�B��D����Һ��ϣ����ɰ�ɫ�������������������E��Һ�������ݲ�����ͬʱ���а�ɫ�������ڣ���ϵ�������пɵó�B��D����Һ������ɰ�ɫ����ΪBaCO3�����������E��Һ��ɫ����ת��ΪBaSO4����B�к�CO32-��D�к�Ba2+��C�к�Ca2+������CaCO3��BaCO3��������ˮ�����BΪNa2CO3��Ca2+����C1O-��C1-�����CΪCaC12��Ca��C1O��2��
��������A��E��Һ����ɫ��Ӧ���ʻ�ɫ��˵��A��E����Na+����AΪNaClO��EΪNaHSO4��
�ʴ�Ϊ��NaC1O��Na2CO3��CaC12��Ca��C1O��2��NaHSO4��
��2��EΪNaHSO4����ˮ��Һ�е��룺NaHSO4�TNa++H++SO42-��H+������������Ӧ��6H++2Al�T2Al3++H2�����ʹ۲쵽����Ϊ�����������
�ʴ�Ϊ�������������
��3��B��ҺΪNa2CO3��Һ��E��ҺΪNaHSO4��Һ����B����Һ�л�����������ϡ��E��Һ��������Ӧ��
Na2CO3+NaHSO4�TNaHCO3+Na2SO4������Ҫ�����ʳɷ�ΪNaHCO3��Na2SO4��
�ʴ�Ϊ��Na2SO4��NaHCO3��
��4��D�к�Ba2+���ڿ����г��ھ��û�ʧЧ��˵��D���ȶ�����ȷ��DΪBa��ClO��2���Ϳ����еĶ�����̼��ˮ������Ӧ�������Ȼ����ʹ����ᣬ�������ټ���ֽ��ʧЧ������ʽΪ��Ba��ClO��2+CO2+H2O�TBaCl2+2HClO��2HClO
 2HCl+O2����
2HCl+O2�����ʴ�Ϊ��Ba��ClO��2+CO2+H2O�TBaCl2+2HClO��2HClO
 2HCl+O2����
2HCl+O2������������1�����ݲ�����Ʒ����Һ��ɫ��ȥ���Ƶ�A������������ӣ���ϵ�����ܼ����Ƶ�A�����ݲ����ڡ��۶����ɰ�ɫ��������ȷ��B��̼������ӣ����B����ˮ��ȷ����B�����ɰ�ɫ����������E��Һ���Ƶ�E������������ӣ��������а�ɫ����������E��Һ���ɵ�C�������ӣ����������ɰ�ɫ�������������������E��Һ�������ݲ�����ͬʱ���а�ɫ�������ڣ���ȷ��D�������ӣ�����A��B��C��D��E��Ϊ������ˮ�Ĺ��壬������ӹ��漴���жϳ������ʣ�
��2��EΪNaHSO4��Һ�������H+������H+���������ķ�Ӧ�ó�����
��3��BΪNa2CO3��EΪNaHSO4�����ݷ����ķ�Ӧ��д�������T�ɣ�
��4��D�к�Ba2+���ڿ����г��ھ��û�ʧЧ�����뵽Ư�۵�ʧЧԭ������֪D��ClO-���ο�Ư�۵�ʧЧԭ��д������ʽ��
���������⽫���ӷ�Ӧ��Ԫ�ؼ��仯����֪ʶ�ں���һ�飬��Ҫ��ǿ���ۺ�˼ά������ͨ��ȫ�������ץסϸ�ڣ�������룬��ѵ��˼ά���а�����

��ϰ��ϵ�д�
�����Ŀ