��Ŀ����
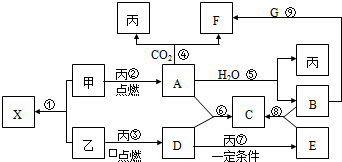
�ס��ҡ���Ϊ�������ʣ��ҡ�����Ԫ�������ڱ���λ��ͬ���塣X��A��B��C��D��E��F��G��Ϊ�����Ļ��������A��X��Ħ��������ͬ��A��F��C��G����ɫ��Ӧ��Ϊ��ɫ����һ�������£��������ת����ϵ����ͼ��

��ش�
��1���û�ѧʽ��ʾ����Ϊ___________��EΪ ____________��
��2��X�ĵ���ʽΪ_____________��
��3��A��H2O��Ӧ�Ļ�ѧ����ʽΪ____________��
��4��д�����Ӧ�����ӷ�Ӧ����ʽ______________��
��5��д���������ȼ����D�Ļ�ѧ��Ӧ����ʽ���������ת�������______________��
��1���û�ѧʽ��ʾ����Ϊ___________��EΪ ____________��
��2��X�ĵ���ʽΪ_____________��
��3��A��H2O��Ӧ�Ļ�ѧ����ʽΪ____________��
��4��д�����Ӧ�����ӷ�Ӧ����ʽ______________��
��5��д���������ȼ����D�Ļ�ѧ��Ӧ����ʽ���������ת�������______________��
��1��O2 �� SO3
��2��
��3��2Na2O2+2H2O==4NaOH+O2
��4��HCO3- + OH- = H2O + CO32-
��5��S+O2 SO2
SO2
��2��

��3��2Na2O2+2H2O==4NaOH+O2
��4��HCO3- + OH- = H2O + CO32-
��5��S+O2
 SO2
SO2 
��ϰ��ϵ�д�
 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�
�����Ŀ