��Ŀ����
 �Ը������������Ĺ�ҵ��ҺΪԭ�������������Ĺ������£����ֲ����������ԣ���
�Ը������������Ĺ�ҵ��ҺΪԭ�������������Ĺ������£����ֲ����������ԣ��� (1 )�ӷ�Һ���ᴿ���ᾧ��FeSO4��7H2O��
(1 )�ӷ�Һ���ᴿ���ᾧ��FeSO4��7H2O�� (2)��
(2)�� ��Һ���Թ�����
��Һ���Թ����� ��Һ��ϣ��õ���
��Һ��ϣ��õ��� ����Һ
����Һ (3 )����Һ���ˣ���90��C��ˮϴ�ӳ����������õ�
(3 )����Һ���ˣ���90��C��ˮϴ�ӳ����������õ� ����
���� (4)����
(4)���� ���õ�
���õ� ����
���� ��֪��
��֪�� ����ˮ�зֽ�
����ˮ�зֽ� (1 )�У�����������м��ȥ��Һ�е�
(1 )�У�����������м��ȥ��Һ�е� ���÷�Ӧ�����ӷ���ʽ��
���÷�Ӧ�����ӷ���ʽ��  (2)�У����һ�������ᣬ���û�ѧƽ��ԭ���������������
(2)�У����һ�������ᣬ���û�ѧƽ��ԭ���������������  (3 )�У�����
(3 )�У����� �����ӷ���ʽ�� ����
�����ӷ���ʽ�� ���� ��Һ��ʱ�䱩¶�ڿ����У����в��ֹ�������Ϊ���ɫ���ñ仯�Ļ�ѧ����ʽ�� ��
��Һ��ʱ�䱩¶�ڿ����У����в��ֹ�������Ϊ���ɫ���ñ仯�Ļ�ѧ����ʽ�� �� (4)�У�ͨ������
(4)�У�ͨ������ ���жϳ����Ƿ�ϴ�Ӹɾ�������
���жϳ����Ƿ�ϴ�Ӹɾ������� �������� ��
�������� �� ��5����֪����
��5����֪���� �Ļ�ѧ����ʽ��
�Ļ�ѧ����ʽ�� ��������464.0kg
��������464.0kg
�� ���õ�316.8kg��Ʒ������Ʒ������ֻ��
���õ�316.8kg��Ʒ������Ʒ������ֻ�� ����ò�Ʒ��
����ò�Ʒ�� �������� kg��Ħ������/g��
�������� kg��Ħ������/g�� ��
�� ��
��
(1)Fe + 2Fe3+ = 3Fe2+
 (2)�������ᣬH+Ũ������ʹFe2+ + 2H2O
(2)�������ᣬH+Ũ������ʹFe2+ + 2H2O Fe(OH)2 + 2H+��ƽ�����淴Ӧ�����ƶ����Ӷ�����FeSO4��ˮ��
Fe(OH)2 + 2H+��ƽ�����淴Ӧ�����ƶ����Ӷ�����FeSO4��ˮ��
 ��3��Fe2+ + 2HCO3- = FeCO3��+ CO2�� + H2O
��3��Fe2+ + 2HCO3- = FeCO3��+ CO2�� + H2O
 4FeCO3 + 6H2O + O2 = 4Fe(OH)3��+ 4CO2
4FeCO3 + 6H2O + O2 = 4Fe(OH)3��+ 4CO2
 ��4��ȡ����ϴ�Ӻ����Һ�����Թ��У��μ��ữ��BaCl2��Һ�����ް�ɫ���������������ϴ�Ӹɾ�
��4��ȡ����ϴ�Ӻ����Һ�����Թ��У��μ��ữ��BaCl2��Һ�����ް�ɫ���������������ϴ�Ӹɾ�
 ��5��288.0
��5��288.0
����

 ����
����
 I �ӷ�Һ���ᴿ���ᾧ��FeSO4��7H2O��
I �ӷ�Һ���ᴿ���ᾧ��FeSO4��7H2O�� ��Һ���Թ�����
��Һ���Թ����� ��Һ��ϣ��õ���
��Һ��ϣ��õ��� ����Һ
����Һ IV ����Һ���ˣ���90��C��ˮϴ�ӳ����������õ�
IV ����Һ���ˣ���90��C��ˮϴ�ӳ����������õ�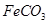 ����
���� ����
����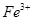 ���÷�Ӧ�����ӷ���ʽ��
���÷�Ӧ�����ӷ���ʽ��
 ���жϳ����Ƿ�ϴ�Ӹɾ�������
���жϳ����Ƿ�ϴ�Ӹɾ������� ��֪����
��֪���� ��������464.0kg��
��������464.0kg�� ����ò�Ʒ��
����ò�Ʒ�� ��������
kg��Ħ������/g��
��������
kg��Ħ������/g�� ��
�� ��
��