��Ŀ����
ʳ��Ϊ��������ƴ����֣����ұ��涨����ʳ�����Ậ�����õ���3.5g/100mL��
��1��ij�о�С�����õζ��������ⶨijƷ��ʳ���д���ĺ���������˵����ȷ����______��
a����NaOH��Һ�ζ�ʱ��Ӧ�����ӷ���ʽΪ��H++OH-=H2O
b��ʳ��������ϡ��һ���������ٽ��еζ�
c����NaOH��Һ�ζ�ʳ�ף���ʹ�÷�̪�������ָʾ��
d������ø�Ʒ��ʳ�����ʵ���Ũ��Ϊ0.75mol?L-1�����ʳ��������Ϊ4.5/100mL��
��2���о�С���ͬѧ��ϸ�۲��˸�Ʒ��ʳ�ı�ǩ���������л����б���������ΪʳƷ���Ӽ������������Ϸ���֤������ʳƷ���Ӽ�����������C6H5COONa�����ᷢ�����ӻ�����Ӧ���������һ���¶��µĴ����뱽�����______����д��ţ���
a��pH����b������ȡ���c�����볣������d���ܽ��
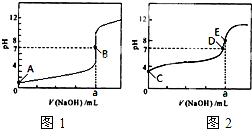
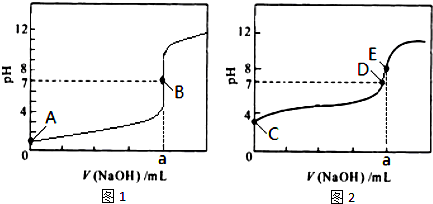
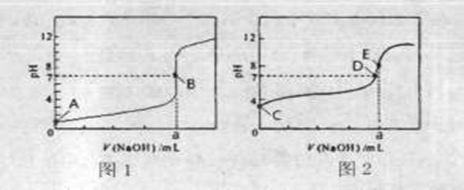
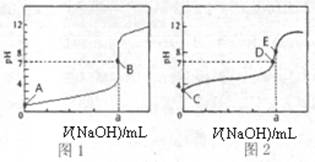
��3�������£���0.1000mol?L-1NaOH��Һ�ֱ�ζ�20.00mL 0.1000mol?L-1HCl��Һ��20.00mL 0.1000mol?L-1CH3COOH��Һ���õ�2���ζ����ߣ���ͼ��ʾ��

�ٵζ�������Һ��������______���ͼl����ͼ2�������ζ�������a=______mL��
��E��pH��8��ԭ�������ӷ���ʽ��ʾ______��
��4���������Բⶨһδ֪Ũ�ȵ�CH3COOH��Һ�ĵ���ȣ�Ӧ����ʵ���������Լ�����ֽ����______��
a����⣬NaOH��Һ����b������Na2CO3��Һ
c��������Ӧ��ʯ����Һ����d���к͵ζ���pH��ֽ��
�⣺��1��a������Ϊ������ʣ����ӷ���ʽӦΪCH3COOH+OH-=H2O����a����
b��ʳ��������ϡ��һ���������ٽ��еζ���Ŀ��Ϊ��Сʵ������b��ȷ��
c������Ϊ������ʣ��ζ��յ�ʱ����Һ�ʼ��ԣ������ü���Ϊ���ָʾ�����������ϴ�c����
d��ʳ�����ʵ���Ũ��Ϊ0.75mol?L-1��������Ϊ0.75mol?L-1��60g/mol=45g/L����4.5g/100mL����d��ȷ��
�ʴ�Ϊ��bd��
��2����Һ��Ũ�Ȳ�ͬ��pH��ͬ����ȷ�����Ե�ǿ����������̶�����ҺŨ�ȵ�Ӱ�죬һ�㲻���ڱȽ����Ե�ǿ�����ܽ�������������ʣ������Ե�ǿ���أ�һ���¶��£����볣��Խ������Խǿ���ʴ�Ϊ��c��
��3���ٴ���Ϊ������ʣ��ζ�������pH�仯�����Ỻ�����ζ��յ�ʱ��Һ�ʼ��ԣ���ζ�������Һ��������ͼ2���ζ��յ�ʱn��CH3COOH��=n��NaOH������a=20.00mL���ʴ�Ϊ��ͼ2��20.00��
�ڴ�����Ϊǿ�������Σ���Һ�ʼ��ԣ�ԭ����CH3COO-+H2O?CH3COOH+OH-���ʴ�Ϊ��CH3COO-+H2O?CH3COOH+OH-��
��4�������Բⶨһδ֪Ũ�ȵ�CH3COOH��Һ�ĵ���ȣ�Ӧ�ֱ�ⶨc��CH3COOH����c��H+������ͨ���к͵ζ�ȷ��c��CH3COOH����ͨ��pH��ֽ�ⶨc��H+�����ʴ�Ϊ��d��
��������1��a������Ϊ������ʣ����ӷ���ʽ��ӦдΪ��ѧʽ��
b��ʵ��ʱ��Ϊ��С��Ӧ������Һ��Ũ�ȣ�
c�������ü���Ϊ���ָʾ����
d��ʳ�����ʵ���Ũ��Ϊ0.75mol?L-1��������Ϊ0.75mol?L-1��60g/mol=45g/L����4.5g/100mL��
��2��һ���¶��£����볣��Խ������Խǿ��
��3������Ϊ������ʣ��ζ��յ�ʱ����Һ�ʼ��ԣ�
��4�������Բⶨһδ֪Ũ�ȵ�CH3COOH��Һ�ĵ���ȣ�Ӧ�ֱ�ⶨc��CH3COOH����c��H+����
�����������ۺϿ������ʵĺ����ⶨ������ϵĶ����жϺͼ����Լ�������ʵĵ���������ˮ���֪ʶ����Ŀ�Ѷ��еȣ�����Ϣ���ϴ���ʱע�����⣮
b��ʳ��������ϡ��һ���������ٽ��еζ���Ŀ��Ϊ��Сʵ������b��ȷ��
c������Ϊ������ʣ��ζ��յ�ʱ����Һ�ʼ��ԣ������ü���Ϊ���ָʾ�����������ϴ�c����
d��ʳ�����ʵ���Ũ��Ϊ0.75mol?L-1��������Ϊ0.75mol?L-1��60g/mol=45g/L����4.5g/100mL����d��ȷ��
�ʴ�Ϊ��bd��
��2����Һ��Ũ�Ȳ�ͬ��pH��ͬ����ȷ�����Ե�ǿ����������̶�����ҺŨ�ȵ�Ӱ�죬һ�㲻���ڱȽ����Ե�ǿ�����ܽ�������������ʣ������Ե�ǿ���أ�һ���¶��£����볣��Խ������Խǿ���ʴ�Ϊ��c��
��3���ٴ���Ϊ������ʣ��ζ�������pH�仯�����Ỻ�����ζ��յ�ʱ��Һ�ʼ��ԣ���ζ�������Һ��������ͼ2���ζ��յ�ʱn��CH3COOH��=n��NaOH������a=20.00mL���ʴ�Ϊ��ͼ2��20.00��
�ڴ�����Ϊǿ�������Σ���Һ�ʼ��ԣ�ԭ����CH3COO-+H2O?CH3COOH+OH-���ʴ�Ϊ��CH3COO-+H2O?CH3COOH+OH-��
��4�������Բⶨһδ֪Ũ�ȵ�CH3COOH��Һ�ĵ���ȣ�Ӧ�ֱ�ⶨc��CH3COOH����c��H+������ͨ���к͵ζ�ȷ��c��CH3COOH����ͨ��pH��ֽ�ⶨc��H+�����ʴ�Ϊ��d��
��������1��a������Ϊ������ʣ����ӷ���ʽ��ӦдΪ��ѧʽ��
b��ʵ��ʱ��Ϊ��С��Ӧ������Һ��Ũ�ȣ�
c�������ü���Ϊ���ָʾ����
d��ʳ�����ʵ���Ũ��Ϊ0.75mol?L-1��������Ϊ0.75mol?L-1��60g/mol=45g/L����4.5g/100mL��
��2��һ���¶��£����볣��Խ������Խǿ��
��3������Ϊ������ʣ��ζ��յ�ʱ����Һ�ʼ��ԣ�
��4�������Բⶨһδ֪Ũ�ȵ�CH3COOH��Һ�ĵ���ȣ�Ӧ�ֱ�ⶨc��CH3COOH����c��H+����
�����������ۺϿ������ʵĺ����ⶨ������ϵĶ����жϺͼ����Լ�������ʵĵ���������ˮ���֪ʶ����Ŀ�Ѷ��еȣ�����Ϣ���ϴ���ʱע�����⣮

��ϰ��ϵ�д�
 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д�
�����Ŀ