��Ŀ����
����Ŀ���л�������G�Ǻϳ�ά������ҩ����м��壬��ϳ�·�����£�

����A��F�ֱ����һ���л�������ϳ�·���в��ֲ��P��Ӧ��������ȥ��֪��

GΪ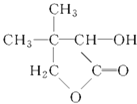 ��
��
��ش��������⣺
��1��G�ķ���ʽ_____________��D�й����ŵ�������_________��
��2����������Ӧ�Ļ�ѧ����ʽΪ__________________________��
��3����������Ӧ�Ļ�ѧ����ʽΪ__________________________��
��4��д��F�Ľṹ��ʽ_____________��
��5������~������Ӧ�����ڼӳɷ�Ӧ����___________________������ȡ����Ӧ����
__________________________�����������
��6��ͬʱ��������������E��ͬ���칹����_____________�֡�
��ֻ��һ�ֹ����ţ�
����״�ṹ���ޡ�O��O����
���˴Ź�������ֻ��2��塣
���𰸡���1��C6H10O3������ȩ�����ǻ���д��©д��д�������÷�
��2��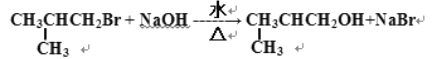
��3��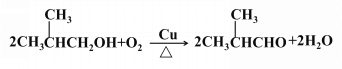
��4��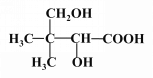
��5���٢������ڢ� ��©д��һ�֣�д�����÷��� ��6��3
��������
����������춡ϩ���廯�ⷢ���ӳɷ�Ӧ���������A��A���������Ƶ�ˮ��Һ����ȡ����Ӧ���ɴ�B��B���������������춡ȩ����B��2-��-1-������A��2-��-1-����飬�춡ȩ��C��Ӧ����D��Dˮ�������Ҵ���E�����������Ϣ֪��E�����������ӳɷ�Ӧ����F��F���ȷֽ�����ˮ��G������G�Ľṹ��ʽ֪��F�Ľṹ��ʽΪ��HOCH2 C��CH3��2CHOHCOOH��E�Ľṹ��ʽΪ��OHCC��CH3��2CHOHCOOH��D�Ľṹ��ʽΪ��OHCC��CH3��2CHOHCOOCH2CH3��C�Ľṹ��ʽΪ��OHCCOOCH2CH3��
��1������G�Ľṹ��ʽ֪��G�ķ���ʽΪC6H10O3��D�Ľṹ��ʽΪOHCC��CH3��2CHOHCOOCH2CH3�����еĹ�������������ȩ�����ǻ���
��2��������±����������ˮ����Ӧ�Ļ�ѧ����ʽΪ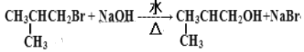 ��
��
��3����������Ӧ�Ļ�ѧ����ʽΪ![]() ��
��
��4��д��F�Ľṹ��ʽ��HOCH2 C��CH3��2CHOHCOOH��
��5������~������Ӧ�����Ǽӳɷ�Ӧ������ȡ����Ӧ������������Ӧ�����ӳɷ�Ӧ����ȡ����Ӧ�����ӳɷ�Ӧ���������ڼӳɷ�Ӧ�����٢ܢ���ȡ����Ӧ�����ڢ���
��6��ͬʱ����������E��ͬ���칹���У�CH3COOCH2CH2OOCCH3��CH3CH2OOCCOOCH2CH3��CH3OOCCH2CH2COOCH3 ����3�֡�

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�����Ŀ����ˮú���ǹ�ҵ�ϳɰ���ԭ����������Ҫ�ɷ���H2��CO��CO2��N2��H2O��g������ˮú���������в���ת��Ϊ�ϳɰ���ԭ�ϡ�

���������գ�
��1����ˮú�������������⡣����ˮú����Ʒͨ��____��Һ������д�Լ�������������_______������֤����������ڡ�
��2����ˮú����ͭ����ʵ��CO�任��CO+H2O![]() CO2+H2
CO2+H2
����ˮú����V��H2��:V��CO��:V��N2��=38��28��22����CO�任��������У�V��H2��:V��N2��=____________��
��3����Һ���շ����ѳ�������̼�ķ���֮һ����֪��
Na2CO3 | K2CO3 | |
20����Һ���Ũ����mol/L�� | 2.0 | 8.0 |
��ļ۸���Ԫ/kg�� | 1.25 | 9.80 |
��ѡ��Na2CO3��Һ������Һ�����ŵ���__________��ȱ����____________�����ѡ��K2CO3��Һ������Һ����ʲô�������Խ��ͳɱ���
___________________________________________
д�����ַ����漰�Ļ�ѧ��Ӧ����ʽ��_______________________
��4�������Dzⶨ��ˮú����H2�Լ�CO�����������ʵ�鷽����
ȡһ���������״�����İ�ˮú������������ʵ�鲽��ⶨ����H2�Լ�CO�����������

��ѡ�ú��ʵ����Լ��ֱ������������������������С�
����ʵ�鷽���У�������������Ŀ���ǣ� ��
����ʵ�鷽���У�����________��ѡ����������������������ȷ����ˮú����H2�����������


