��Ŀ����
(1)A��B��C����ѧ��ѧ������������ɫ���ʣ������Ԫ�ؾ���������Ԫ�أ���Ħ�����������������Ǿ�����ˮ����������ԭ��Ӧ����ˮ�Ȳ���������Ҳ���ǻ�ԭ������д��A��B��C��ˮ��Ӧ�Ļ�ѧ����ʽ��
A+H2O________________�� B+H2O__________________ C+H2O__________________
(2)D��E��F�Ƕ�����Ԫ����ɵ����ʣ�D��ˮ��Ӧ�����У�ˮ����������E��ˮ��Ӧ�����У�ˮ�ǻ�ԭ����F��ˮ�������ֽⷴӦ����д��D��E��F��ˮ��Ӧ�Ļ�ѧ����ʽ��
D+H2O_________________�� E+H2O_________________�� F+H2O________________��
 HCl+HClO��2Na2O2+2H2O=4NaOH+O2��
HCl+HClO��2Na2O2+2H2O=4NaOH+O2��(2)2Na+2H2O=2NaOH+H2����2F2+2H2O=4HF+O2��Al2S3+6H2O=2Al(OH)3��+3H2S��

 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д� ����������ϵ�д�
����������ϵ�д���1��ˮ��������ԭ���ڻ�̬ʱ��������Ų�ʽΪ
��2��H2O��������ԭ�Ӳ�ȡ����
��3��ˮ�������õ�һ��H+�γ�ˮ�������ӣ�H3O+�������������̵�������������������
A����ԭ�ӵ��ӻ����ͷ����˸ı� B��������״�����˸ı�
C��ˮ�����Ա������Ļ�ѧ���� D�����еļ��Ƿ����˸ı�
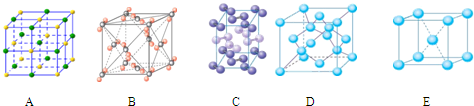
��4���������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ͼ��δ��˳����������ľ���
������ͬ����
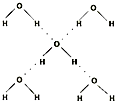
��5���ڱ������У�ÿ��ˮ���������ڵ�4��ˮ�����γ��������ͼ��ʾ������֪������������51kJ/mol��������⣬ˮ���Ӽ仹���ڷ��»�����11kJ/mol�����������������ġ����ܡ���
��6������ɫ����ˮCuSO4�ܽ���ˮ�У���Һ����ɫ������Ϊ������һ�ֳ���ɫ��������ӣ���д�����ɴ�������ӵ����ӷ���ʽ��
��7����֪����Ԫ�صĵ縺�����ݣ�H��2.1��O��3.5��F��4.0��OF2��ˮ������ṹ���ƣ���ˮ���ӵļ��Ա�OF2ǿ�ö࣬��ԭ���У���OF2����ԭ���������Թ¶Ե��ӣ�������FһO���й��õ��Ӷ�ƫ��F�������ļ��ԣ��ڴӵ縺���Ͽ���
��8�������±����ݣ���д�������߸����ԵĽ��ۣ�
| ���� | ���� ��kJ/mol�� |
���� ��pm�� |
���� | ���� | ���� | �۵㣨�棩 | �е㣨�棩 |
| H-C | 413 | 109 | 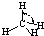 |
109.5�� | ���� | -183.7 | -128.0 |
| H-N | 391 | 101 | 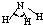 |
107�� | �� | -77.7 | -33.3 |
| H-O | 467 | 96 | 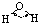 |
104.5�� | ˮ | 0.0 | 100.0 |
ˮ������֮Դ��Ҳ��һ�ֳ��õ��Լ�����ش��������⣺
��1��ˮ��������ԭ���ڻ�̬ʱ��������Ų�ʽΪ___ _______��
��2��H2O��������ԭ�Ӳ�ȡ���� �ӻ���
��3��ˮ�������õ�һ��H���γ�ˮ�������ӣ�H3O���������������̵������������������� ��
| A����ԭ�ӵ��ӻ����ͷ����˸ı� | B��������״�����˸ı� |
| C��ˮ�������ӷ��ӹ����������� | D�����еļ��Ƿ����˸ı� |
ˮ������֮Դ��Ҳ��һ�ֳ��õ��Լ�����ش��������⣺
��1��ˮ��������ԭ���ڻ�̬ʱ��������Ų�ʽΪ ��
��2��H2O��������ԭ�Ӳ�ȡ���� �ӻ���
��3��ˮ�������õ�һ��H���γ�ˮ�������ӣ�H3O���������������̵������������������� ��
A����ԭ�ӵ��ӻ����ͷ����˸ı� B�����Ŀռ乹�ͷ����˸ı�
C�����еļ��Ƿ����˸ı�
��4���������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ͼ(δ��˳������)������ľ���������ͬ����______��(������Ӧ�ı����д)
 |

��5���ڱ������У�ÿ��ˮ���������ڵ�4��ˮ�����γ����(��ͼ��ʾ)����֪������������51 kJ/mol��������⣬ˮ���Ӽ仹���ڷ��»���(11 kJ/mol)���������������ġ����ܡ���_________kJ/mol��
��6������ɫ����ˮCuSO4�ܽ���ˮ�У���Һ����ɫ������Ϊ������һ�ֳ���ɫ��������ӡ���д�����ɴ�������ӵ����ӷ���ʽ�� ��

