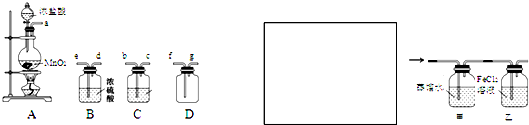
��Ŀ����
��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��100g5.00%��NaOH��Һ��������CuSO4��Һ��100g
10.00%��K2SO4��Һ���缫��Ϊʯī�缫��
10.00%��K2SO4��Һ���缫��Ϊʯī�缫��

��1����ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������c�缫�������ӡ�
�ݴ˻ش����⣺
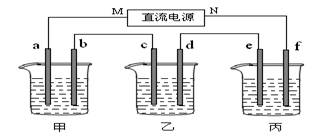
�� ��Դ��N��Ϊ___________����
�� �缫b�Ϸ����ĵ缫��ӦΪ___________��
�� ��ʽ����缫b�����ɵ������ڱ�״���µ������___________��
�� �缫c�������仯��___________g��
�� ���ǰ�����Һ���ᡢ���Դ�С�Ƿ����仯��������ԭ��
����Һ____________________������Һ___________________������Һ______________________��
��2�������������ͭȫ����������ʱ����ܷ�������У�Ϊʲô��____________________________________________��
�ݴ˻ش����⣺
�� ��Դ��N��Ϊ___________����
�� �缫b�Ϸ����ĵ缫��ӦΪ___________��
�� ��ʽ����缫b�����ɵ������ڱ�״���µ������___________��
�� �缫c�������仯��___________g��
�� ���ǰ�����Һ���ᡢ���Դ�С�Ƿ����仯��������ԭ��
����Һ____________________������Һ___________________������Һ______________________��
��2�������������ͭȫ����������ʱ����ܷ�������У�Ϊʲô��____________________________________________��
��1������������4OH--4e-==2H2O+O2������2.8L����16g���ݼ�������Ϊ�൱�ڵ��ˮ���Ҽ�С��OH-
�ŵ磬H+���ࣻ�����䣬�൱�ڵ��ˮ
��2�����ԣ���ΪCuSO4��Һ��ת��ΪH2SO4��Һ����ӦҲ�ͱ�Ϊˮ�ĵ�ⷴӦ
�ŵ磬H+���ࣻ�����䣬�൱�ڵ��ˮ
��2�����ԣ���ΪCuSO4��Һ��ת��ΪH2SO4��Һ����ӦҲ�ͱ�Ϊˮ�ĵ�ⷴӦ

��ϰ��ϵ�д�
�����Ŀ
��12�֣�A��B��C����ǿ����ʣ�������ˮ�е�������������±���ʾ��
| ������ | Na+��K+��Cu2+ |
| ������ | SO42����OH�� |


��ͨ��Դ������һ��ʱ��������c�缫����������16g�������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ���ϡ��ݴ˻ش��������⣺
��1��MΪ��Դ�� ������д�������������缫b�Ϸ����ĵ缫��ӦΪ ��
��2������缫e�����ɵ������ڱ�״̬�µ������ ��
��3��д�����ձ��ĵ��ط�Ӧ
��4�������������B��Һ�еĽ�������ȫ����������ʱ����ܷ�������У�Ϊʲô��
��5��������һ��ʱ��������c�缫����������16g��Ҫʹ���ָ���ԭ����״̬�������� ��