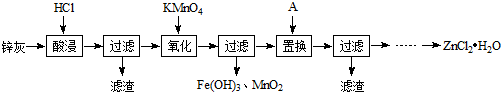
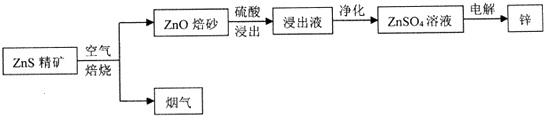
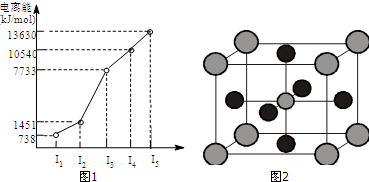
��Ŀ����
4�� ��ͼ��ʾ��Zn��Cu�γɵ�ԭ��أ�ijʵ����ȤС������ʵ����ڶ��鿨�ϵļ�¼���£���Ƭ�ϵ������������ǣ�������
��ͼ��ʾ��Zn��Cu�γɵ�ԭ��أ�ijʵ����ȤС������ʵ����ڶ��鿨�ϵļ�¼���£���Ƭ�ϵ������������ǣ���������CuΪ������ZnΪ����
��Cu���������ݲ�����������ԭ��Ӧ
��SO${\;}_{4}^{2-}$��Cu���ƶ�
������0.5mol�����������ߣ���ɲ���0.25mol����
�ݵ��ӵ������ǣ�Cu��Zn
��������Ӧʽ��Cu+2e-�TCu2+������������Ӧ��
| A�� | �٢ڢ� | B�� | �ڢ� | C�� | �ڢۢ� | D�� | �ۢܢ� |
���� ��ԭ����У�Zn��ʧ������������Cu�������������Ϸ���������Ӧ�������Ϸ�����ԭ��Ӧ�����ӴӸ����ص��������������������Һ���������������ƶ������������ƶ����ݴ˷������
��� �⣺�ٸ�ԭ����У�п��ʧ������������Cu���������ʴ���
��Cu���������ӵõ����������������������ݲ�����������ԭ��Ӧ������ȷ��
�۷ŵ�ʱ���������Һ���������������ƶ������������ƶ�������SO${\;}_{4}^{2-}$��Zn���ƶ����ʴ���
�ܸ���2H++2e-=H2��֪������0.5mol�����������ߣ������������ʵ���=$\frac{0.5mol}{2}��1$=0.25mol������ȷ��
�ݷŵ�ʱ������Znʧ���ӡ�����Cu�õ��ӣ����Ե��ӵ������ǣ�Zn��Cu���ʴ���
�������������ӵõ��������������缫��Ӧʽ��2H++2e-=H2����������ԭ��Ӧ���ʴ���
��ѡB��
���� ���⿼��ԭ���ԭ������ȷ�������жϡ��缫��Ӧʽ����Ӧ���͵�֪ʶ�㼴�ɽ���״������ж���Һ�����������ƶ�������Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
14�����ж��ڷ�Ӧ3NO2+H2O=2HNO3+NO��˵������ȷ���ǣ�������
| A�� | �������뻹ԭ����������Ϊ1��2 | |
| B�� | NO2��������ˮ�ǻ�ԭ�� | |
| C�� | ����1molNO����6mol���ӷ���ת�� | |
| D�� | �������뻹ԭ�������ʵ�����Ϊ2��1 |
15����1mol/L�ģ�NH4��2SO4��Һ�У������й�����Ũ�ȴ�С�Ƚ��У���ȷ���ǣ�������
| A�� | c��NH4+��=2c��SO42-����c��H+��=c��OH-�� | B�� | c��NH4+����c��SO42-����c��H+����c��OH-�� | ||
| C�� | c��SO42-����c��NH4+����c��H+����c��OH-�� | D�� | c��SO42-����c��NH4+����c��OH-����c��H+�� |
12������������ȷ���ǣ�������
| A�� | ������ѧ��Ӧʱʧȥ����Խ��Ľ���ԭ�ӣ���ԭ����Խǿ | |
| B�� | ���������ӱ���ԭ��һ���õ���Ԫ�صĵ��� | |
| C�� | �������������ͬ��ԭ�ӣ�һ����ͬ��Ԫ�ص�ԭ�� | |
| D�� | ���Ϸ�Ӧ���û���Ӧ������������ԭ��Ӧ |
9�� �¶�ΪTʱ����2.0L�����ܱ������г���1.0mol PCl5����ӦPCl5��g��?PCl3��g��+Cl2��g������һ��ʱ���ﵽƽ�⣮��Ӧ�����вⶨ�IJ���������ͼ������˵����ȷ���ǣ�������
�¶�ΪTʱ����2.0L�����ܱ������г���1.0mol PCl5����ӦPCl5��g��?PCl3��g��+Cl2��g������һ��ʱ���ﵽƽ�⣮��Ӧ�����вⶨ�IJ���������ͼ������˵����ȷ���ǣ�������
 �¶�ΪTʱ����2.0L�����ܱ������г���1.0mol PCl5����ӦPCl5��g��?PCl3��g��+Cl2��g������һ��ʱ���ﵽƽ�⣮��Ӧ�����вⶨ�IJ���������ͼ������˵����ȷ���ǣ�������
�¶�ΪTʱ����2.0L�����ܱ������г���1.0mol PCl5����ӦPCl5��g��?PCl3��g��+Cl2��g������һ��ʱ���ﵽƽ�⣮��Ӧ�����вⶨ�IJ���������ͼ������˵����ȷ���ǣ�������| A�� | ��Ӧ��ǰ50 s ��ƽ������v��PCl3��=0.0032 mol•L-1•s-1 | |
| B�� | ���������������䣬�����¶ȣ�ƽ��ʱc��PCl3��=0.11mol•L-1����Ӧ�ġ�H��0 | |
| C�� | ��ͬ�¶��£���ʼʱ�������г���2.0 mol PCl3��2.0mol Cl2���ﵽƽ��ʱ��PCl3 ��ת����С��80% | |
| D�� | ��ͬ�¶��£���ʼʱ�������г���1.0 mol PCl5��0.20 mol PCl3 ��0.20 mol Cl2����Ӧ�ﵽƽ��ǰv��������v���棩 |