��Ŀ����
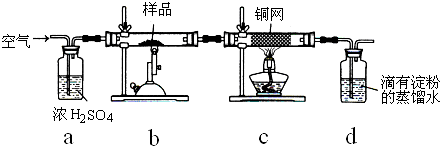
ijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飬ʵ��ʱ��ҩƷA��μ��뵽����B�У����������ʵ��ش����⣺
��1����AΪˮ��BΪ�������ƣ�C��ʢ���ữ����FeCl2��Һ����������E��C�е�����Ϊ______��
C�з�����Ӧ�����ӷ���ʽΪ______��
��2����������װ�û�������֤���ʵ����ʣ������֤�������ԣ�KMnO4��Cl2������ȡƯ��Һ����A�м�Ũ���ᣬB�м�______��Һ��C�з�����Ӧ�����ӷ���ʽΪ______��
��3����������װ�û�������֤SO2�Ļ�ѧ���ʣ�AΪ���ᣬBΪ�������ƹ��壬��ôC��ʢ��______��Һʱ����֤�仹ԭ�ԣ� ��C��ʢ��______��Һʱ����֤��Ư���ԣ�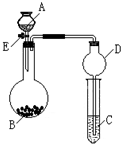
��1����AΪˮ��BΪ�������ƣ�C��ʢ���ữ����FeCl2��Һ����������E��C�е�����Ϊ______��
C�з�����Ӧ�����ӷ���ʽΪ______��
��2����������װ�û�������֤���ʵ����ʣ������֤�������ԣ�KMnO4��Cl2������ȡƯ��Һ����A�м�Ũ���ᣬB�м�______��Һ��C�з�����Ӧ�����ӷ���ʽΪ______��
��3����������װ�û�������֤SO2�Ļ�ѧ���ʣ�AΪ���ᣬBΪ�������ƹ��壬��ôC��ʢ��______��Һʱ����֤�仹ԭ�ԣ� ��C��ʢ��______��Һʱ����֤��Ư���ԣ�

��1������������ˮ��Ӧ�������������������л�ԭ�Ե�FeCl2�����ɻ�ɫ��Fe3+��������Ӧ�����ӷ���ʽΪ4Fe2++O2+4H+=4Fe3++2H2O��
�ʴ�Ϊ����Һ��dz��ɫ��Ϊ���أ���ɫ��4Fe2++O2+4H+=4Fe3++2H2O��
��2��KMnO4��Cl2�����Ը��������Ũ���ᷴӦ����������B��Ӧ���������أ��Ʊ�Ư��Һ����������NaOH��Һ��Ӧ����Ӧ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+H2O��
�ʴ�Ϊ��������أ����Ը�����أ���Cl2+2OH-=Cl-+ClO-+H2O��
��3��SO2���л�ԭ�ԣ�������ˮ�����������ʷ�Ӧ��SO2����Ư���ԣ���ʹƷ����ɫ������C��Ӧʢ��Ʒ�죬
�ʴ�Ϊ����ˮ������ˮ�����Ը��������Һ����Ʒ�죮
�ʴ�Ϊ����Һ��dz��ɫ��Ϊ���أ���ɫ��4Fe2++O2+4H+=4Fe3++2H2O��
��2��KMnO4��Cl2�����Ը��������Ũ���ᷴӦ����������B��Ӧ���������أ��Ʊ�Ư��Һ����������NaOH��Һ��Ӧ����Ӧ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+H2O��
�ʴ�Ϊ��������أ����Ը�����أ���Cl2+2OH-=Cl-+ClO-+H2O��
��3��SO2���л�ԭ�ԣ�������ˮ�����������ʷ�Ӧ��SO2����Ư���ԣ���ʹƷ����ɫ������C��Ӧʢ��Ʒ�죬
�ʴ�Ϊ����ˮ������ˮ�����Ը��������Һ����Ʒ�죮

��ϰ��ϵ�д�
�����Ŀ
 ����Ժǿ��������ˮ���������̱����ֽ�ˮ�����ۺ���̬���������빤�̽�����Э������ˮ��Դ��������Ϊ���ߣ��ѽ�ˮ�����ۡ���̬�����������ˮ��Ϊһ��������ϵͳ��������ԭ���ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ�������������ij������Һ�У����д�����Mg2+��Al3+��Cu2+��Ag+���Է����ش��������⣺
����Ժǿ��������ˮ���������̱����ֽ�ˮ�����ۺ���̬���������빤�̽�����Э������ˮ��Դ��������Ϊ���ߣ��ѽ�ˮ�����ۡ���̬�����������ˮ��Ϊһ��������ϵͳ��������ԭ���ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ�������������ij������Һ�У����д�����Mg2+��Al3+��Cu2+��Ag+���Է����ش��������⣺ ijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飬ʵ��ʱ��ҩƷA��μ��뵽����B�У����������ʵ��ش����⣺
ijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飬ʵ��ʱ��ҩƷA��μ��뵽����B�У����������ʵ��ش����⣺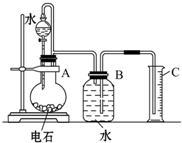 ijͬѧ�������ͼ��ʾ��ʵ��װ�������Եزⶨ��ʯ��̼���Ƶ�����������
ijͬѧ�������ͼ��ʾ��ʵ��װ�������Եزⶨ��ʯ��̼���Ƶ�����������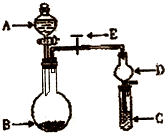 ��2010?����һģ����ѧʵ����һ��װ������������ɶ��ʵ�飬Aijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飮��ش�
��2010?����һģ����ѧʵ����һ��װ������������ɶ��ʵ�飬Aijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飮��ش�