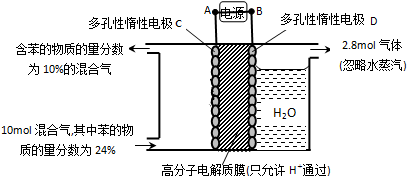
��Ŀ����
14�������ʾΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã���ش��������⣺| �� ���� | IA | 0 | ||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | ||||
��2���͢ߵ���ۺ����������ǿ��ΪHClO4��H2SO4 ������Ļ�ѧʽ��ʾ����
��3���١�������Ԫ�ذ�ԭ�Ӹ���֮��Ϊ1��1��ɵij���Һ̬�������������Һ���ܽ�Fe 2+������д���÷�Ӧ�����ӷ���ʽH2O2+2Fe2++2H+=2Fe3++2H2O��
��4���ɱ���Ԫ���γɵ����ʿɷ�����ͼ�еķ�Ӧ������B��C��G�ǵ��ʣ�BΪ����ɫ���壬D��Һ�Լ��ԣ�

��д��D��Һ��G��Ӧ�Ļ�ѧ����ʽ2Al+2NaOH+2H2O=2NaAlO2+3H2����
��д������A��Һ�����ʵ������ӵķ���ȡ����A��Һ�μӼ��Σ�ϡ�����ữ����������Һ�а�ɫ�������ɣ�
�۳����£������1L0.1mol•L-1��A��Һ��һ��ʱ�������ҺpHΪ12��������Һ����仯������õ�������ת�Ƶ��ӵ����ʵ���Ϊ0.01mol��
��д��������E��F��Һ�����ʷ�Ӧ�����ӷ���ʽAlO2-+4H+=Al3++2H2O��
��5���ɢڡ��ܡ���Ԫ����ɵĻ�����W����������Һ�з��ƣ�W��ԭ�Խ�ǿ��������Һ���ױ��ߵĵ����������÷�Ӧ�����ӷ���ʽΪS2O32-+4Cl2+5H2O�T2SO42-+8Cl-+10H+��
���� ����Ԫ�������ڱ��е�λ�ã���֪��ΪH����ΪO����ΪF����ΪNa����ΪAl����ΪS����ΪCl��
��1��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����
��2��ͬ�����������Ԫ�طǽ�������ǿ���ǽ�����Խǿ����ۺ����������Խǿ��
��3���١�������Ԫ�ذ�ԭ�Ӹ���֮��Ϊ1��1��ɵij���Һ̬������ΪH2O2����������Һ���ܽ�Fe2+����Fe3+��ͬʱ����ˮ��
��4���ɱ���Ԫ���γɵ����ʣ�����B��C��G�ǵ��ʣ�BΪ����ɫ���壬��BΪCl2��D��Һ�Լ��ԣ����A��ҺΪ����Ȼ�����Һ�����������������������ƣ���DΪNaOH��CΪH2����EΪHCl��G��������������Һ��Ӧ�õ��õ�������F����GΪAl��FΪNaAlO2������������NaAlO2��Ӧ�����Ȼ������Ȼ��ƺ�ˮ��
��5���ɢڡ��ܡ���Ԫ����ɵĻ�����W����������Һ�з��ƣ�W��ԭ�Խ�ǿ������WΪNa2S2O3����������Һ��Na2S2O3��������������ԭ�������ɣ���Һ���ƣ�����Һ���ױ���������������������������ӣ�
��� �⣺����Ԫ�������ڱ��е�λ�ã���֪��ΪH����ΪO����ΪF����ΪNa����ΪAl����ΪS����ΪCl��
��1��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶��Na��Cl��F��
�ʴ�Ϊ��Na��Cl��F��
��2���ǽ�����Cl��S���ǽ�����Խǿ����ۺ����������Խǿ�������ԣ�HClO4��H2SO4��
�ʴ�Ϊ��HClO4��H2SO4��
��3���١�������Ԫ�ذ�ԭ�Ӹ���֮��Ϊ1��1��ɵij���Һ̬������ΪH2O2����������Һ���ܽ�Fe2+����Fe3+��ͬʱ����ˮ����Ӧ���ӷ���ʽΪ��H2O2+2Fe2++2H+=2Fe3++2H2O��
�ʴ�Ϊ��H2O2+2Fe2++2H+=2Fe3++2H2O��
��4���ɱ���Ԫ���γɵ����ʣ�����B��C��G�ǵ��ʣ�BΪ����ɫ���壬��BΪCl2��D��Һ�Լ��ԣ����A��ҺΪ����Ȼ�����Һ�����������������������ƣ���DΪNaOH��CΪH2����EΪHCl��G��������������Һ��Ӧ�õ��õ�������F����GΪAl��FΪNaAlO2����
��D��Һ��G��Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ڼ���NaCl��Һ�����ʵ������ӵķ�����ȡ����A��Һ�μӼ��Σ�ϡ�����ữ����������Һ�а�ɫ�������ɣ�
�ʴ�Ϊ��ȡ����A��Һ�μӼ��Σ�ϡ�����ữ����������Һ�а�ɫ�������ɣ�
�۳����£������1L 0.1mol/L��NaCl��Һ��һ��ʱ�������Һ�е�c��OH -��=10-2mol/L����n��NaOH��=0.01mol/L��1L=0.01mol����2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2������֪������Ϊ0.01mol��$\frac{1}{2}$=0.005mol����ת�Ƶ������ʵ���=0.005mol��2=0.01mol��
�ʴ�Ϊ��0.01mol��
�ܹ�����E��F��Һ�����ʷ�Ӧ�����ӷ���ʽΪAlO2-+4H+=Al3++2H2O��
�ʴ�Ϊ��AlO2-+4H+=Al3++2H2O��
��5���ɢڡ��ܡ���Ԫ����ɵĻ�����W����������Һ�з��ƣ�W��ԭ�Խ�ǿ������WΪNa2S2O3����������Һ��Na2S2O3��������������ԭ�������ɣ���Һ���ƣ�����Һ���ױ���������������������������ӣ�����Һ����������Ӧ�����ӷ���ʽΪS2O32-+4Cl2+5H2O�T2SO42-+8Cl-+10H+��
�ʴ�Ϊ��S2O32-+4Cl2+5H2O�T2SO42-+8Cl-+10H+��
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɡ�������ƶϣ��Ѷ��еȣ�ע�����������йص����ӷ���ʽ����д��������ԭ��Ӧ�ķ���ʽ����д����Ҫѧ���߱���ʵ�Ļ�����

| A�� | �Ҷ����ͱ�������1��1 | B�� | �Ҵ����Ҷ�����1��2 | ||
| C�� | �״����Ҵ���5��1 | D�� | �״����Ҵ���4��1 |
| A�� | ȡ��ȡҺ����������AgNO3��Һ�а�ɫ��������������ܺ���Cl- | |
| B�� | ȡ��ȡҺ����������Cu��ŨH2SO4���Թܿ��к���ɫ�������������ܺ���NO3- | |
| C�� | ȡ��ȡҺ���������������ữ��BaCl2��Һ���а�ɫ������������һ����SO42- | |
| D�� | �ýྻ�IJ�˿��պȡ��ȡҺ���ھƾ������������գ���ɫ�ʻ�ɫ����һ������Na+ |
| A�� |  ��ȡ�������������� | B�� |  ʯ�͵����� | ||
| C�� |  ��ȥCO�����е�CO2���� | D�� |  ����HCl |
| A�� | ������������Z��R��Y | |
| B�� | ��̬�⻯����ȶ��ԣ�Y��Z | |
| C�� | R��X���������Ϊ���ӻ����� | |
| D�� | X��Y��������������Ӧ��ˮ���������Ӧ |
| A�� | ��Ҫ���ȵĻ�ѧ��Ӧ�������ȷ�Ӧ | |
| B�� | ˮ�������ǽ���ѧ��ת��Ϊ���ܵĹ��� | |
| C�� | ʳ�ס����ʳ�ηֱ������ᡢ��� | |
| D�� | ����������֡���ͭ�����ںϽ� |
������������Һ�м������ᣬ��Ӧ���ң����������ΪpH��ͬ��ϡ���ᣬ��ʼʱ��Ӧ�������Ժ���������ٶȽϿ죬�ٶȱ仯��ԭ���ǣ�������
| A�� | �ݳ�ClO2ʹ������Ũ�Ƚ��� | B�� | ��ʹHClO2�ķֽ���� | ||
| C�� | ��Һ�е�H+������� | D�� | ��Һ�е�Cl-������� |
| A�� | 1��1��3 | B�� | 1��2��3 | C�� | 1��3��3 | D�� | 3��2��2 |
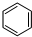 ��g��+3H2��g��$\frac{\underline{\;\;\;\;\;\;\;\;\;����\;\;\;\;\;\;\;\;\;}}{Fe_{3}O_{4}/Al_{2}O_{3}}$
��g��+3H2��g��$\frac{\underline{\;\;\;\;\;\;\;\;\;����\;\;\;\;\;\;\;\;\;}}{Fe_{3}O_{4}/Al_{2}O_{3}}$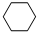 ��g��
��g��