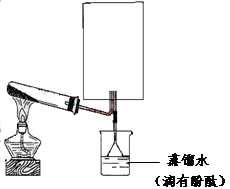
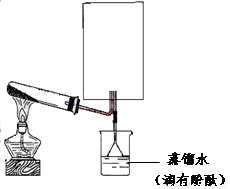
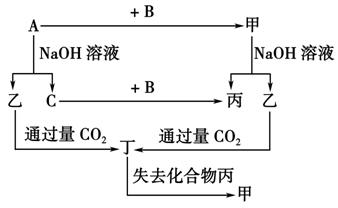
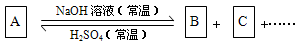
��Ŀ����
(10�֣���ͼ��Ԫ�����ڱ��Ŀ�ܣ�����a��b��c��d��e��f ����Ԫ�ص�λ�á�
![]()

![]() �ݴ˻ش��������⣺
�ݴ˻ش��������⣺
(1������ʵ���������Ԫ�����ڱ��н���Ԫ����ǽ���Ԫ�صķֽ��ߡ�
(2) a��b��c��d ����Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳��������___________________��
(3����a��c��f ����Ԫ���γɵ�ǿ��Ļ�ѧʽ��______________________��
(4������������Ԫ���У���������ǿ���ǣ���Ԫ�ط��ţ�_________������Ԫ��λ��Ԫ�����ڱ��е�____________��___________���С�
( 5 ) b ��c �γɵĻ�����ĵ���ʽΪ_____________________________��
( 6 )A������Ԫ�ض��Ƿǽ���Ԫ�� B������Ԫ�ض��ǽ���Ԫ��
C���ǽ���Ԫ�ض�λ�ڶ����� D���ǽ���Ԫ�ض����ڸ���
(7����a��c�γɵ�һ�ֻ�������c��e �γɵ�һ�ֻ������������ԭ��Ӧ������һ��ǿ�ᣬ��д���÷�Ӧ�Ļ�ѧ����ʽ____________________________________________��
��1����
��2��d>b>c>a
��3��HClO4 ����HClO3��
��4��Na 3 12
![]()
��6��D
��7��SO2 +H2O2 ��H2SO4