��Ŀ����
��ͼ��ʾ��X��Y�ֱ���ֱ����Դ��������ͨ�����a�����������ӣ�b���崦����ɫ��������ų���������һ�������
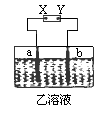
[����]
| �� |
a���� |
b���� |
X�缫 |
Z��Һ |
|
A�� |
п |
ʯī |
���� |
CuSO4 |
|
B�� |
ʯī |
ʯī |
���� |
NaOH |
|
C�� |
�� |
�� |
���� |
AgNO3 |
|
D�� |
ͭ |
ʯī |
���� |
CuCl2 |
�𰸣�A

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д�
�����Ŀ
 ��֪X��Y��Z����Ԫ����ɵĻ����������Ӿ��壬�侧����ͼ��ʾ��X��Y��Z�ֱ���������Ķ��㡢��ߵ��е㡢����������ģ��������ʾ�û�����Ļ�ѧʽ��ȷ�ģ�������
��֪X��Y��Z����Ԫ����ɵĻ����������Ӿ��壬�侧����ͼ��ʾ��X��Y��Z�ֱ���������Ķ��㡢��ߵ��е㡢����������ģ��������ʾ�û�����Ļ�ѧʽ��ȷ�ģ�������


