��Ŀ����
ij�¶�ʱ����2L�ܱ���������̬����CO��H2��Ӧ������̬����Z�����ǵ����ʵ�����ʱ��ı仯���±���ʾ��| t/min | CO/mol | H2/mol | Z/mol |
| 1.00 | 1.00 | 0.00 | |
| 1 | 0.90 | 0.80 | 0.10 |
| 3 | 0.75 | 0.50 | 0.25 |
| 5 | 0.65 | 0.30 | 0.35 |
| 7 | 0.55 | 0.10 | 0.45 |
| 9 | 0.55 | 0.10 | 0.45 |
| 10 | 0.55 | 0.10 | 0.45 |
��2����ϵ�з�����Ӧ�Ļ�ѧ����ʽ��______��
��3����3-5minʱ���ڲ���Z��ƽ����Ӧ���ʣ�______��

���𰸡���������1���ɱ������ݿ�֪����ʼCO�����ʵ���Ϊ1mol����CO����ʼŨ��Ϊ =0.5mol/L��Z����ʼŨ��Ϊ0��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol����CO��ƽ��Ũ��Ϊ
=0.5mol/L��Z����ʼŨ��Ϊ0��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol����CO��ƽ��Ũ��Ϊ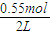 =0.275mol/L��ƽ��ʱZ�����ʵ���Ϊ0.45mol����Z��ƽ��Ũ��Ϊ
=0.275mol/L��ƽ��ʱZ�����ʵ���Ϊ0.45mol����Z��ƽ��Ũ��Ϊ =0.225mol/L���ݴ���ͼ��
=0.225mol/L���ݴ���ͼ��
��2���ɱ������ݿ�֪��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol��H2�����ʵ���Ϊ0.1mol��ƽ��ʱZ�����ʵ���Ϊ0.45mol����������ʵ����ʵ����仯�����������ʵ����仯��֮�ȵ��ڻ�ѧ������֮�ȼ�������ʵ�����ѧ��������������ԭ���غ��ж�Z�ķ���ʽ���ݴ���д��
��3���ɱ������ݿ�֪3-5minʱ���ڲ���Z�����ʵ����仯��Ϊ0.35mol-0.25mol=0.1mol������v=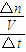 ����v��Z����
����v��Z����
����⣺��1���ɱ������ݿ�֪����ʼCO�����ʵ���Ϊ1mol����CO����ʼŨ��Ϊ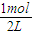 =0.5mol/L��Z����ʼŨ��Ϊ0��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol����CO��ƽ��Ũ��Ϊ
=0.5mol/L��Z����ʼŨ��Ϊ0��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol����CO��ƽ��Ũ��Ϊ =0.275mol/L��ƽ��ʱZ�����ʵ���Ϊ0.45mol����Z��ƽ��Ũ��Ϊ
=0.275mol/L��ƽ��ʱZ�����ʵ���Ϊ0.45mol����Z��ƽ��Ũ��Ϊ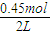 =0.225mol/L����CO��Z�����ʵ�����Ũ�ȣ�c����ʱ�䣨t���仯������Ϊ��
=0.225mol/L����CO��Z�����ʵ�����Ũ�ȣ�c����ʱ�䣨t���仯������Ϊ��
 ��
��
�ʴ�Ϊ��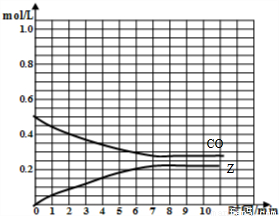 ��
��
��2���ɱ������ݿ�֪��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol���ʡ�n��CO��=1mol-0.55mol=0.45mol��H2�����ʵ���Ϊ0.1mol����n��H2��=1mol-0.1mol=0.9mol��ƽ��ʱZ�����ʵ���Ϊ0.45mol����CO��H2��Z�Ļ�ѧ������֮��Ϊ0.45mol��0.9mol��0.45mol=1��2��1����CO+2H2=Z����Z�ķ���ʽΪCH4O���ṹʽΪCH3OH���ʸ÷�ӦΪCO+2H2?CH3OH��
�ʴ�Ϊ��CO+2H2?CH3OH��
��3���ɱ������ݿ�֪3-5minʱ���ڲ���Z�����ʵ����仯��Ϊ0.35mol-0.25mol=0.1mol��
��v��Z��= =0.025 mol/��L?min����
=0.025 mol/��L?min����
�ʴ�Ϊ��0.025 mol/��L?min����
���������鷴Ӧ���ʼ��㡢��ѧƽ���йؼ��㡢Ũ����ʱ��仯ͼ��ȣ��Ѷ��еȣ�ע����ͼ�������ƽ����Ũ�ȡ�����ƽ���ʱ�䣮
 =0.5mol/L��Z����ʼŨ��Ϊ0��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol����CO��ƽ��Ũ��Ϊ
=0.5mol/L��Z����ʼŨ��Ϊ0��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol����CO��ƽ��Ũ��Ϊ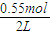 =0.275mol/L��ƽ��ʱZ�����ʵ���Ϊ0.45mol����Z��ƽ��Ũ��Ϊ
=0.275mol/L��ƽ��ʱZ�����ʵ���Ϊ0.45mol����Z��ƽ��Ũ��Ϊ =0.225mol/L���ݴ���ͼ��
=0.225mol/L���ݴ���ͼ����2���ɱ������ݿ�֪��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol��H2�����ʵ���Ϊ0.1mol��ƽ��ʱZ�����ʵ���Ϊ0.45mol����������ʵ����ʵ����仯�����������ʵ����仯��֮�ȵ��ڻ�ѧ������֮�ȼ�������ʵ�����ѧ��������������ԭ���غ��ж�Z�ķ���ʽ���ݴ���д��
��3���ɱ������ݿ�֪3-5minʱ���ڲ���Z�����ʵ����仯��Ϊ0.35mol-0.25mol=0.1mol������v=
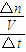 ����v��Z����
����v��Z��������⣺��1���ɱ������ݿ�֪����ʼCO�����ʵ���Ϊ1mol����CO����ʼŨ��Ϊ
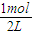 =0.5mol/L��Z����ʼŨ��Ϊ0��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol����CO��ƽ��Ũ��Ϊ
=0.5mol/L��Z����ʼŨ��Ϊ0��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol����CO��ƽ��Ũ��Ϊ =0.275mol/L��ƽ��ʱZ�����ʵ���Ϊ0.45mol����Z��ƽ��Ũ��Ϊ
=0.275mol/L��ƽ��ʱZ�����ʵ���Ϊ0.45mol����Z��ƽ��Ũ��Ϊ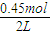 =0.225mol/L����CO��Z�����ʵ�����Ũ�ȣ�c����ʱ�䣨t���仯������Ϊ��
=0.225mol/L����CO��Z�����ʵ�����Ũ�ȣ�c����ʱ�䣨t���仯������Ϊ�� ��
���ʴ�Ϊ��
 ��
����2���ɱ������ݿ�֪��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol���ʡ�n��CO��=1mol-0.55mol=0.45mol��H2�����ʵ���Ϊ0.1mol����n��H2��=1mol-0.1mol=0.9mol��ƽ��ʱZ�����ʵ���Ϊ0.45mol����CO��H2��Z�Ļ�ѧ������֮��Ϊ0.45mol��0.9mol��0.45mol=1��2��1����CO+2H2=Z����Z�ķ���ʽΪCH4O���ṹʽΪCH3OH���ʸ÷�ӦΪCO+2H2?CH3OH��
�ʴ�Ϊ��CO+2H2?CH3OH��
��3���ɱ������ݿ�֪3-5minʱ���ڲ���Z�����ʵ����仯��Ϊ0.35mol-0.25mol=0.1mol��
��v��Z��=
 =0.025 mol/��L?min����
=0.025 mol/��L?min�����ʴ�Ϊ��0.025 mol/��L?min����
���������鷴Ӧ���ʼ��㡢��ѧƽ���йؼ��㡢Ũ����ʱ��仯ͼ��ȣ��Ѷ��еȣ�ע����ͼ�������ƽ����Ũ�ȡ�����ƽ���ʱ�䣮

��ϰ��ϵ�д�
 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ
 ij�¶�ʱ����2L�ܱ���������̬����X��Y��Ӧ������̬����Z�����ǵ����ʵ�����ʱ��ı仯�����ʾ��
ij�¶�ʱ����2L�ܱ���������̬����X��Y��Ӧ������̬����Z�����ǵ����ʵ�����ʱ��ı仯�����ʾ�� 2Z
2Z ij�¶�ʱ����2L�ܱ������У�A��B��C�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ����ͼ�����ݷ�����
ij�¶�ʱ����2L�ܱ������У�A��B��C�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ����ͼ�����ݷ����� 2C��g��
2C��g��
 2Z
2Z
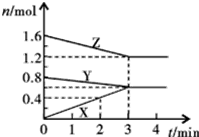 ij�¶�ʱ����2L�ܱ������У�X��Y��Z������̬���ʵ����ʵ�����ʱ��仯
ij�¶�ʱ����2L�ܱ������У�X��Y��Z������̬���ʵ����ʵ�����ʱ��仯