��Ŀ����
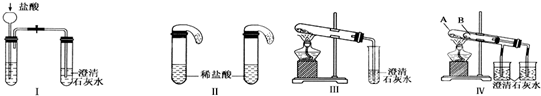
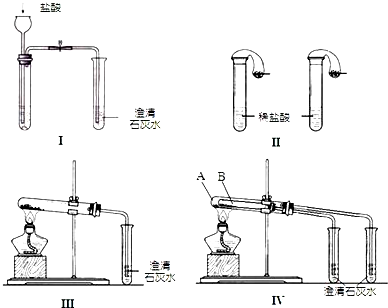
ijУ��ѧ����С��Ϊ�˼���̼���ƺ�̼���������ְ�ɫ���壬�ò�ͬ�ķ�����������ʵ�飬��ͼI-IV��ʾ��
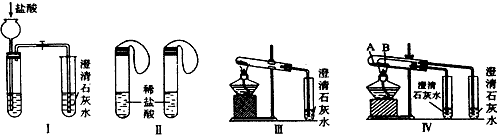
��1��ֻ����ͼI��II��ʾʵ�飬�ܹ����õĴﵽʵ��Ŀ���ǣ���װ����ţ�__________��
��2��ͼIII��IV��ʾʵ����ܼ������������ʣ��䷴Ӧ�Ļ�ѧ����ʽΪ _______________________����ʵ��III��ȣ�ʵ��IV���ŵ��ǣ���ѡ����ţ�_______________��
A��IV��III����
B��IV��III��ȫ
C��IV��III�������
D��IV����������һ��װ��ͬʱ���������Ա�ʵ�飬��III���С���
��3������ʵ��IV��֤̼���ƺ�̼�����Ƶ��ȶ��ԣ����Թ�B��װ��Ĺ��������______________������
��4����̼��������Һ�����ʯ��ˮ��ϲ���ַ�Ӧ��
�ٵ�ʯ��ˮ����ʱ�������ӷ���ʽΪ___________________ ��
�ڵ�̼�������������������ʵ���֮��Ϊ2 : 1ʱ��������Һ�����ʵĻ�ѧʽΪ_________�������ʵ�����������Һ�����ʵ�������______________��
��2��ͼIII��IV��ʾʵ����ܼ������������ʣ��䷴Ӧ�Ļ�ѧ����ʽΪ _______________________����ʵ��III��ȣ�ʵ��IV���ŵ��ǣ���ѡ����ţ�_______________��
A��IV��III����
B��IV��III��ȫ
C��IV��III�������
D��IV����������һ��װ��ͬʱ���������Ա�ʵ�飬��III���С���
��3������ʵ��IV��֤̼���ƺ�̼�����Ƶ��ȶ��ԣ����Թ�B��װ��Ĺ��������______________������
��4����̼��������Һ�����ʯ��ˮ��ϲ���ַ�Ӧ��
�ٵ�ʯ��ˮ����ʱ�������ӷ���ʽΪ___________________ ��
�ڵ�̼�������������������ʵ���֮��Ϊ2 : 1ʱ��������Һ�����ʵĻ�ѧʽΪ_________�������ʵ�����������Һ�����ʵ�������______________��
��1����
��2��2NaHCO3 Na2CO3+CO2��+H2O��D
Na2CO3+CO2��+H2O��D
��3��NaHCO3��
��4����HCO3-+OH-+Ca2+==CaCO3��+H2O����Na2CO3������Һ�еμ��Ȼ�����Һ�����˳��ij����м����ᣬ�������ܽ⣬������������ʹ����ʯ��ˮ����ǣ���֤����̼�������
��2��2NaHCO3
 Na2CO3+CO2��+H2O��D
Na2CO3+CO2��+H2O��D ��3��NaHCO3��
��4����HCO3-+OH-+Ca2+==CaCO3��+H2O����Na2CO3������Һ�еμ��Ȼ�����Һ�����˳��ij����м����ᣬ�������ܽ⣬������������ʹ����ʯ��ˮ����ǣ���֤����̼�������

��ϰ��ϵ�д�
�����Ŀ
 ijУ��ѧ����С��Ϊ�˼���̼���ƺ�̼���������ְ�ɫ���壬�ò�ͬ�ķ�����������ʵ�飬��ͼ����ʾ��
ijУ��ѧ����С��Ϊ�˼���̼���ƺ�̼���������ְ�ɫ���壬�ò�ͬ�ķ�����������ʵ�飬��ͼ����ʾ��