��Ŀ����
����Ŀ����Ȼ�ĺ;����˹��Ʊ��ľ��嶼���ڸ���ȱ�ݣ�������ij��������NiO�����оʹ�������ͼ��ʾ��ȱ�ݣ�һ��Ni2+��ȱ����������Ni2+������Ni3+��ȡ�������������Գʵ����ԣ����˻�������Ni��O�ı�ֵȴ�����˱仯��ij��������Ʒ����и�����Ni��O��97��100
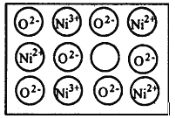
(1)д������������Ʒ�Ļ�ѧʽ_________________��
(2)�Լ��㾧����Ni2+��Ni3+�����Ӹ���֮��__________________��
���𰸡�Ni0.97O 91:6
��������
(1)ij��������Ʒ����и�����Ni��O��97��100���仯ѧʽ�ɱ�ʾΪNi97O100���������������Ļ�ѧʽ��ʽ��д����ɱ�ʾΪNi0.97O����Ϊ��Ni0.97O��
(2)��n(Ni2+)=x����n(Ni3+)=97-x�����ݵ���غ㣬2x+3(97-x)=2��100���Ӷ����x=91����97-x=6��������Ni2+��Ni3+�����Ӹ���֮��Ϊ91:6����Ϊ��91:6��

��ϰ��ϵ�д�
�����Ŀ


