��Ŀ����
��14�֣�ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
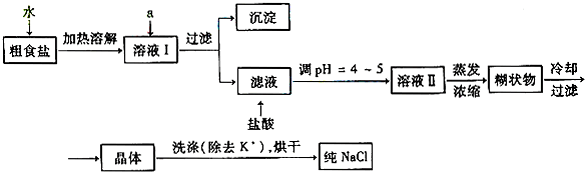
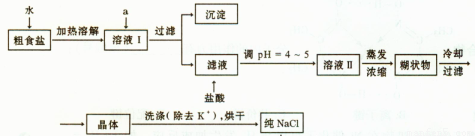
��1����ʳ�γ���������K+��Ca2+��Mg2+��Fe3+��SO2-4'���������ӣ�ʵ�����ᴿNaCl���������£�
�ṩ���Լ�������Na2CO3��Һ ����K2CO3��Һ NaOH��Һ BaCl2��Һ Ba��NO3��2 ��Һ 75%�Ҵ� ���Ȼ�̼
������ȥ��ҺI�е�Ca2+��Mg2+��Fe3+��SO2-4���ӣ�ѡ��a���������Լ������μ�˳������Ϊ ���ѧʽ������Һ�еμ������pH =4 ~5��Ŀ���� ��
��ѡ��75%�Ҵ�ϴ�ӳ�ȥNaCl������渽��������KCl������NaCl�Ƿ�ϴ���IJ����� ��
��2�����ᴿ��NaCl����480mL 4.00mol��L-1NaCl��Һ����Ҫ��ȡ������Ϊ g������������ҩ�ס����������ձ���� �����������ƣ���
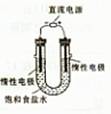
��3����ⱥ��ʳ��ˮ��װ����ͼ��ʾ�����ռ���H2Ϊ2L����ͬ���������ռ���Cl2____���>������=����<����2L����Ҫԭ���� ��
��1����BaCl2����NaOH��Na2CO3����NaOH��BaCl2��Na2CO3 ��BaCl2����Na2CO3 ��
NaOH��2�֣�����ѡ����ѡ���÷֣����к���Һ�й�����OH-��CO32-����2�֣���2���ø�
���IJ�˿ȡ���һ��ϴ��Һ���ڻ��������գ�����ɫ�ܲ���δ����ɫ����ϴ�ɾ�����2�֣�
��2��117��2�֣�����ƽ��500mL����ƿ����ͷ�ιܣ���2�֣���3��������2�֣�������ɵ�
Cl2��NaOH��Ӧ. ��2�֣�
��������(1)����ȥ������Һ�е�Ca2+��Mg2+��Fe3+��SO42-���ӣ��ֱ���Na2CO3����NaOH��
NaOH ��BaCl2�������Լ�Ҫ�������������Լ��ں��������б��������Na2CO3������
BaCl2������Na2CO3����NaOH�������������ϴ��Һ����K+,��ϴ�ɾ��ˣ����ȡ��
��һ��ϴ��Һ����ɫ��Ӧ���ɣ�(2)����û��480mL����ƿ������ֻ����500mL ��Һ����
0.5x4.00x58.5=117;(3)����Cl2��NaOH��Ӧ��ʹ�ռ���Cl2<2L��