��Ŀ����
����Ŀ�������ȣ�Sr3N2���ڹ�ҵ�Ϲ㷺��������ӫ��ۡ����뵪���ڼ��������¿����ɵ����ȣ���������ˮ���ҷ�Ӧ��ijͬѧ�������װ���Ʊ������ȣ���װ��ʢװ�����Լ�������ʹ�õĵ�����Ʒ���ܺ�������CO��CO2��O2���������ʡ�
��֪�������������ͭCH3COO[Cu(NH3)2]��Һ�ܶ�������CO�����ױ�O2������ʧȥ����CO�������������Ӽ�����Һ�ܶ�������O2��
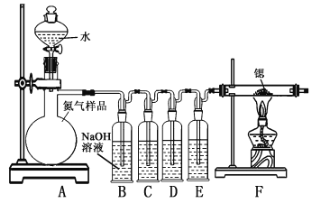
��.�����ȵ���ȡ
(1)װ��B������������_____________��
(2)װ��C��D��Eʢװ���Լ��ֱ���_____________������ţ���
�ף��������Ӽ�����Һ �ң�Ũ���� ���������������ͭ��Һ
(3)����װ����ƴ���ȱ�ݣ����ܻᵼ�²�Ʒ���ʣ�����Ľ�����____________��
��.��Ʒ���ȵIJⶨ
��ȡ6.0 g �������ò�Ʒ��������������ƿ�У�Ȼ���ɺ�ѹ©����������ˮ��ͨ��ˮ�������������İ�ȫ����������200mL1.00mol/L���������Һ��ȫ���գ�����Һ����仯���Բ��ƣ������ձ�����ȡ20.00 mL������Һ����1.00mol/LNaOH����Һ�ζ���ʣ��HCl�����յ�ʱ����16.00mLNaOH��Һ����ͼ�мг�װ���ԣ�
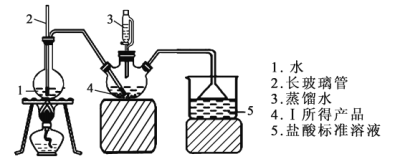
(4)����ƿ�з����Ļ�ѧ��Ӧ����ʽΪ____________________________��
(5)װ����2������Ϊ__________________________________________��
(6)��1.00mol/LNaOH����Һ�ζ���ʣ��HClʱ��ѡָʾ��Ϊ_________������ĸ����
a��ʯ����Һ b����̪��Һ c������
(7)��Ʒ����Ϊ____________________��
(8)����ʵ���������ʹ������(Sr3N2)�ⶨ���ƫ�ߵ���_________������ĸ����
a���ζ�ʱδ��NaOH����Һ��ϴ�ζ���
b������ʱ���ζ�ǰƽ�ӣ��ζ�����
c��ҡ����ƿʱ��Һ�彦��
���𰸡�ϴ��ƿ �ױ��� ��װ��F������һʢ�м�ʯ�ҵĸ���� Sr3N2+6H2O=3Sr(OH)2+2NH3�� ƽ����ѹ����ֹ���� c 96.93% bc
��������
װ��A�ṩ������װ��B��C��D��ȥ������Ʒ���ܺ�������CO2��O2��CO���������ʣ�E���ﵪ����Fװ���Ƿ���װ�ã��ݴ˽��
(1)װ��B����������ϴ��ƿ����Ϊ��ϴ��ƿ��
(2)�����������ͭ��������CO�����ױ�O2���������������Ӽ�����Һ��������O2������Ӧ���ڴ����������ͭ��Һ��ǰ�棬Ũ�������ڸ���N2����������棬��װ��C��D��Eʢװ���Լ��ֱ��Ǽױ��ҡ���Ϊ���ױ��ң�
(3)����װ����ƴ���ȱ�ݣ�ֻ���Ƿ�Ӧǰ���Ӻͷ�ֹˮ�������룬û�п���β�˹ܿڿ��ܻ����������Ӷ��ó��Ľ�����Ϊ��װ��F������һʢ�м�ʯ�ҵĸ���ܡ���Ϊ����װ��F������һʢ�м�ʯ�ҵĸ���ܣ�
(4)����ƿ�з���Sr3N2��H2O��Ӧ����Sr(OH)2��NH3����ѧ����ʽΪSr3N2+6H2O=3Sr(OH)2+2NH3������Ϊ��Sr3N2+6H2O=3Sr(OH)2+2NH3����
(5)װ����2�������ͨ��������ƽ������ѹǿ����ֹ������ѹ��������Ϊ��ƽ����ѹ����ֹ��������Ϊ��ƽ����ѹ����ֹ������
(6)��1.00mol/LNaOH����Һ�ζ���ʣ��HClʱ�����ֹNH4Cl��NaOH������Ӧ������Ӧѡ���ɫ��Χ�����������ָʾ������ѡָʾ��Ϊc����Ϊ��c��
(7)��NH3��Ӧ���������ʵ���Ϊ��0.200L��1.00mol/L-0.0160L��1.00mol/L��![]() =0.04mol��n(Sr3N2)=0.02mol����Ʒ����Ϊ
=0.04mol��n(Sr3N2)=0.02mol����Ʒ����Ϊ ![]() =96.93%����Ϊ��96.93%��
=96.93%������96.93%��
(8)a���ζ�ʱδ��NaOH����Һ��ϴ�ζ��ܣ�����V(NaOH)������NH3��Ӧ��������ʵ�����С����Ʒ����ƫ�ͣ�a�������⣻
b������ʱ���ζ�ǰƽ�ӣ��ζ����ӣ�����V(NaOH)��С����NH3��Ӧ��������ʵ�������Ʒ����ƫ�ߣ�b�������⣻
c��ҡ����ƿʱ��Һ�彦��������V(NaOH)��С����NH3��Ӧ��������ʵ�������Ʒ����ƫ�ߣ�c�������⣻
��ѡbc����Ϊ��bc��

 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�




