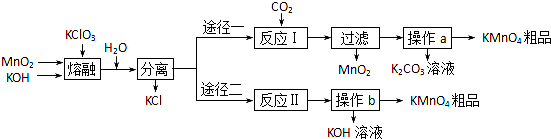
��Ŀ����
ijѧϰС��ͨ���������ϣ�������·����Ʊ�KMnO4��

��֪����3MnO2+KClO3+6KOH 3K2MnO4+KCl+3H2O
3K2MnO4+KCl+3H2O
�ڼ������ʵ��ܽ�ȣ�
| �¶� | �ܽ��/g | ||
| K2CO3 | KOH | KMnO4 | |
| 20�� | 111 | 112 | 6.38 |
| 60�� | 127 | 154 | 22.1 |
A���ƾ��ơ��� B��������������C��������������D�� �ձ�
��2����Ӧ��Ļ�ѧ����ʽ______��
��3����Ӧ�������Ϊ��⣬�仯ѧ����ʽΪ______��
��4������;���в���a��b��ͬ��������______��______�����˵�3���������������п���ѭ�����õIJ�����______��
��5����������1.5mol?L-1�IJ��ᣨH2C2O4����Һ�ζ�KMnO4��Һ���ⶨKMnO4��Ʒ�Ĵ��ȣ�����������������Ҫ�ɼ�������Ϊ______��
�⣺��1�����ڹ���������Ҫ�������ڼ��ȣ����������ﺬ�м���KOHӦ����������������Ҫ�þƾ��ƣ�
��ѡ��AB��
��2����ӦIΪK2MnO4��CO2��Ӧ���ɹ�������ת����ϵ��֪����MnO2��KMnO4��K2CO3����Ӧ����ʽΪ 3K2MnO4+2CO2=MnO2+2KMnO4+2K2CO3��
�ʴ�Ϊ��3K2MnO4+2CO2=MnO2+2KMnO4+2K2CO3��
��3����ӦIIΪ���K2MnO4��Һ���ɹ�������ת����ϵ��֪��������KMnO4��KOH���ɣ�����������ԭ��Ӧ��ˮ�������ŵ磬������H2����ⷴӦʽΪ2K2MnO4+2H2O 2KOH+2KMnO4+H2����
2KOH+2KMnO4+H2����
�ʴ�Ϊ��2K2MnO4+2H2O 2KOH+2KMnO4+H2����
2KOH+2KMnO4+H2����
��4������;���в���a��b��������Һ�л�þ��壬��Ҫ����Ũ������ȴ�ᾧ�����˵ȣ��ʲ���a��bΪ������Ũ������ȴ�ᾧ�����ˣ�
������ͼ���Կ�������Ҫ�����������MnO2��KOH��KClO3��CO2��ˮ�ɲ����ǣ�����Ӧ��������KOH��MnO2����KOH��MnO2�ǿ���ѭ�����õ����ʣ�
�ʴ�Ϊ������Ũ������ȴ�ᾧ��KOH��MnO2��
��5���ɵζ�ʱ���IJ�����Һ��������������ĵIJ�������ʵ��������ݷ���ʽ����n��KMnO4������������m��KMnO4����������ص���������KMnO4��Ʒ�������ɵ�KMnO4��Ʒ�Ĵ��ȣ��ʻ���Ҫ�ɼ�������Ϊ���ζ�ʱ���IJ�����Һ�������KMnO4��Ʒ��������
�ʴ�Ϊ���ζ�ʱ���IJ�����Һ�������KMnO4��Ʒ��������
��������1�����ڹ���������Ҫ�������ڼ��ȣ����������ﺬ�м���KOHӦ����������
��2����ӦIΪK2MnO4��CO2��Ӧ���ɹ�������ת����ϵ��֪����MnO2��KMnO4��K2CO3��
��3����ӦIIΪ���K2MnO4��Һ���ɹ�������ת����ϵ��֪��������KMnO4��KOH���ɣ�����������ԭ��Ӧ��ˮ�������ŵ磬������H2��
��4������;���в���a��bΪ����Һ�л�þ��壬��Ҫ����Ũ������ȴ�ᾧ�����˵ȣ�
��������Ҫ����������ں�������ɸ����ʣ������ʿ���ѭ��ʹ�ã�
��5���ɵζ�ʱ���IJ�����Һ��������������ĵIJ�������ʵ��������ݷ���ʽ����n��KMnO4������������m��KMnO4����������ص���������KMnO4��Ʒ�������ɵ�KMnO4��Ʒ�Ĵ��ȣ�
���������⿼��ѧ���Ķ���Ŀ��ȡ��Ϣ���������ء�ʵ�����������ζ��ȣ��Ѷ��еȣ���2���з���ʽ��дΪ�״��㣬���ݹ��������жϷ�Ӧ����Ȼ�������д����Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ����Ϣ���н�������������
��ѡ��AB��
��2����ӦIΪK2MnO4��CO2��Ӧ���ɹ�������ת����ϵ��֪����MnO2��KMnO4��K2CO3����Ӧ����ʽΪ 3K2MnO4+2CO2=MnO2+2KMnO4+2K2CO3��
�ʴ�Ϊ��3K2MnO4+2CO2=MnO2+2KMnO4+2K2CO3��
��3����ӦIIΪ���K2MnO4��Һ���ɹ�������ת����ϵ��֪��������KMnO4��KOH���ɣ�����������ԭ��Ӧ��ˮ�������ŵ磬������H2����ⷴӦʽΪ2K2MnO4+2H2O
 2KOH+2KMnO4+H2����
2KOH+2KMnO4+H2�����ʴ�Ϊ��2K2MnO4+2H2O
 2KOH+2KMnO4+H2����
2KOH+2KMnO4+H2������4������;���в���a��b��������Һ�л�þ��壬��Ҫ����Ũ������ȴ�ᾧ�����˵ȣ��ʲ���a��bΪ������Ũ������ȴ�ᾧ�����ˣ�
������ͼ���Կ�������Ҫ�����������MnO2��KOH��KClO3��CO2��ˮ�ɲ����ǣ�����Ӧ��������KOH��MnO2����KOH��MnO2�ǿ���ѭ�����õ����ʣ�
�ʴ�Ϊ������Ũ������ȴ�ᾧ��KOH��MnO2��
��5���ɵζ�ʱ���IJ�����Һ��������������ĵIJ�������ʵ��������ݷ���ʽ����n��KMnO4������������m��KMnO4����������ص���������KMnO4��Ʒ�������ɵ�KMnO4��Ʒ�Ĵ��ȣ��ʻ���Ҫ�ɼ�������Ϊ���ζ�ʱ���IJ�����Һ�������KMnO4��Ʒ��������
�ʴ�Ϊ���ζ�ʱ���IJ�����Һ�������KMnO4��Ʒ��������
��������1�����ڹ���������Ҫ�������ڼ��ȣ����������ﺬ�м���KOHӦ����������
��2����ӦIΪK2MnO4��CO2��Ӧ���ɹ�������ת����ϵ��֪����MnO2��KMnO4��K2CO3��
��3����ӦIIΪ���K2MnO4��Һ���ɹ�������ת����ϵ��֪��������KMnO4��KOH���ɣ�����������ԭ��Ӧ��ˮ�������ŵ磬������H2��
��4������;���в���a��bΪ����Һ�л�þ��壬��Ҫ����Ũ������ȴ�ᾧ�����˵ȣ�
��������Ҫ����������ں�������ɸ����ʣ������ʿ���ѭ��ʹ�ã�
��5���ɵζ�ʱ���IJ�����Һ��������������ĵIJ�������ʵ��������ݷ���ʽ����n��KMnO4������������m��KMnO4����������ص���������KMnO4��Ʒ�������ɵ�KMnO4��Ʒ�Ĵ��ȣ�
���������⿼��ѧ���Ķ���Ŀ��ȡ��Ϣ���������ء�ʵ�����������ζ��ȣ��Ѷ��еȣ���2���з���ʽ��дΪ�״��㣬���ݹ��������жϷ�Ӧ����Ȼ�������д����Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ����Ϣ���н�������������

��ϰ��ϵ�д�
 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ
ijѧϰС��ͨ���������ϣ�������·����Ʊ�KMnO4��

��֪����3MnO2+KClO3+6KOH 3K2MnO4+KCl+3H2O
3K2MnO4+KCl+3H2O
�ڼ������ʵ��ܽ�ȣ�
��1����һ����������ʱ�����żܡ������ǡ�ϸ����������ǯ�⣬����������� ��
A���ƾ��� B�������� C�������� D�� �ձ�
��2����Ӧ��Ļ�ѧ����ʽ ��
��3����Ӧ�������Ϊ��⣬�仯ѧ����ʽΪ ��
��4������;���в���a��b��ͬ�������� �� �����˵�3���������������п���ѭ�����õIJ����� ��
��5����������1.5mol?L-1�IJ��ᣨH2C2O4����Һ�ζ�KMnO4��Һ���ⶨKMnO4��Ʒ�Ĵ��ȣ�����������������Ҫ�ɼ�������Ϊ ��

��֪����3MnO2+KClO3+6KOH
 3K2MnO4+KCl+3H2O
3K2MnO4+KCl+3H2O�ڼ������ʵ��ܽ�ȣ�
| �¶� | �ܽ��/g | ||
| K2CO3 | KOH | KMnO4 | |
| 20�� | 111 | 112 | 6.38 |
| 60�� | 127 | 154 | 22.1 |
A���ƾ��� B�������� C�������� D�� �ձ�
��2����Ӧ��Ļ�ѧ����ʽ ��
��3����Ӧ�������Ϊ��⣬�仯ѧ����ʽΪ ��
��4������;���в���a��b��ͬ�������� �� �����˵�3���������������п���ѭ�����õIJ����� ��
��5����������1.5mol?L-1�IJ��ᣨH2C2O4����Һ�ζ�KMnO4��Һ���ⶨKMnO4��Ʒ�Ĵ��ȣ�����������������Ҫ�ɼ�������Ϊ ��