ћвƒњƒЏ»Ё
“їґ®ќ¬ґ»ѕ¬£ђ‘Џ“їЄцєћґ®»Ёїэµƒ√№±’»Ё∆чјпЈҐ…ъN2£®g£©+3H2(g)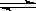 2NH3(g) Јі”¶£ђіЋЈі”¶іпµљїѓ—І∆љЇв„іћђµƒ±к÷Њ «£ЇҐўN2£ђH2ЇЌNH3µƒ÷ ЅњЈ÷ э≤ї‘ўЄƒ±д£ђҐЏ»Ё∆чƒЏµƒ—є«њ≤їЋж ±Љдµƒ±дїѓґш±дїѓ£ђҐџc(N2)°√c(H2)°√c(NH3)£љ1°√3°√2 £ђҐ№µ•ќї ±Љдјп√њ‘цЉ”1molN2£ђЌђ ±‘цЉ”3molH2£ђҐЁ“їґќ ±ЉдƒЏN2£ЇH2£ЇNH3µƒ∆љЊщїѓ—ІЈі”¶Ћў¬ ÷Ѓ±»ќ™1:3:2 £ђҐё»Ё∆ч÷–∆шћеµƒ√№ґ»≤ї±д
2NH3(g) Јі”¶£ђіЋЈі”¶іпµљїѓ—І∆љЇв„іћђµƒ±к÷Њ «£ЇҐўN2£ђH2ЇЌNH3µƒ÷ ЅњЈ÷ э≤ї‘ўЄƒ±д£ђҐЏ»Ё∆чƒЏµƒ—є«њ≤їЋж ±Љдµƒ±дїѓґш±дїѓ£ђҐџc(N2)°√c(H2)°√c(NH3)£љ1°√3°√2 £ђҐ№µ•ќї ±Љдјп√њ‘цЉ”1molN2£ђЌђ ±‘цЉ”3molH2£ђҐЁ“їґќ ±ЉдƒЏN2£ЇH2£ЇNH3µƒ∆љЊщїѓ—ІЈі”¶Ћў¬ ÷Ѓ±»ќ™1:3:2 £ђҐё»Ё∆ч÷–∆шћеµƒ√№ґ»≤ї±д
| A£ЃҐўҐЏҐџҐ№ҐЁҐё | B£ЃҐўҐЏҐЁҐё | C£ЃҐўҐЏҐё | D£Ѓ÷ї”–ҐўҐЏ |
D
љвќц

ЅЈѕ∞≤бѕµЅ–ір∞Є
ѕаєЎћвƒњ
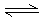 4Z(∆ш)£ЂW(єћ)Јі”¶£ђіпµљ∆љЇв„іћђµƒ±к÷Њ «
4Z(∆ш)£ЂW(єћ)Јі”¶£ђіпµљ∆љЇв„іћђµƒ±к÷Њ «