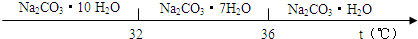��Ŀ����
(1)
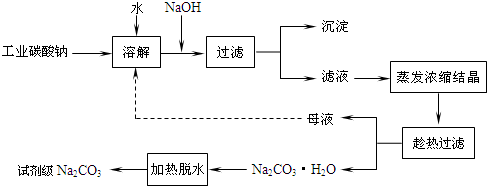
��֪��Na2CO3��10H2O(s)Na2CO3��10H2O(s)![]() Na2CO3��H2O(s)��9H2O(g)����H2����473.63 kJ��mol��1
Na2CO3��H2O(s)��9H2O(g)����H2����473.63 kJ��mol��1
д��Na2CO3��H2O��ˮ�ֽⷴӦ���Ȼ�ѧ����ʽ________��
(2)��֪16 g���嵥��S��ȫȼ��SO2����ʱ���ų�148.4kJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ________��
�𰸣�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ