��Ŀ����
��֪�����Ȼ�ѧ����ʽ����H2(g)��![]() O2(g)
O2(g)![]() H2O(l)����H����285 kJ��mol��1
H2O(l)����H����285 kJ��mol��1
��H2(g)��1/2O2(g)![]() H2O(g)����H����241.8 kJ��mol��1
H2O(g)����H����241.8 kJ��mol��1
��C(s)��1/2O2(g)![]() CO(g)����H����110.5 kJ��mol��1
CO(g)����H����110.5 kJ��mol��1
��C(s)��O2(g)![]() CO2(g)����H����393.5 kJ��mol��1
CO2(g)����H����393.5 kJ��mol��1
�ش��������⣺
(1)H2��ȼ����Ϊ________��C��ȼ����Ϊ________��
(2)ȼ��1 g��H2����Һ̬ˮ���ų�������Ϊ________��
(3)д��COȼ�յ��Ȼ�ѧ����ʽ________��
�𰸣�

��ϰ��ϵ�д�
�����Ŀ
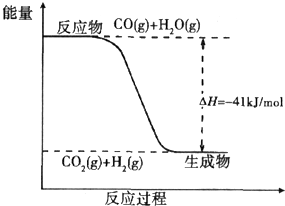 ú̿����ת��Ϊ�����Դ�ͻ���ԭ�ϣ�
ú̿����ת��Ϊ�����Դ�ͻ���ԭ�ϣ�