��Ŀ����
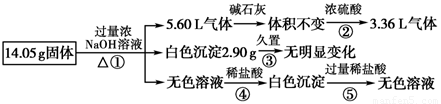
ij�������������Al��(NH4)2SO4��MgCl2��AlCl3��FeCl2�е�һ�ֻ�����ɣ��ֶԸû����������ʵ�飬����������й�������ͼ��ʾ(������������ѻ���ɱ�״���µ����)��
�ش��������⣺
(1)��������Ƿ����FeCl2_______________(��ǡ���)��
(2)��������Ƿ����(NH4)2SO4____________(��ǡ���)��
(3)д����Ӧ�ܵ����ӷ�Ӧʽ��____________________________________��
(4)����ݼ����жϻ�������Ƿ���AlCl3(������ļ������ݺͼ���������Ҫ��д�����������)��
(1)��
(2)��
(3)![]() +H++H2O
+H++H2O![]() Al(OH)3��
Al(OH)3��
(4)��������Ϣ���Ƶ�һ������Al��(NH4)2SO4��MgCl2�������ʣ�����������������ʵ�����֮�պõ���
�������ɢٵ�������֪ԭ�������һ����FeCl2.�ٽ�Ϣڵ�����֪��![]() +H++H2O
+H++H2O![]() Al(OH)3��.
Al(OH)3��.
(4)�������ݿ��Լ���ԭ�������m(Al)=5.4 g��m��(NH4)2SO4��=13.2 g��m(MgCl2)=9.5 g��m(Al)+m��(NH4)2SO4��+m(MgCl2)=

��ϰ��ϵ�д�
�����Ŀ
ij�������������Al����NH4��2SO4��MgCl2��FeCl2��AlCl3�е�һ�ֻ�����ɣ��ֶԸû����������ʵ�飬����������й�������ͼ��ʾ��������������ѻ���ɱ�״���µ��������

����˵����ȷ���ǣ�������

����˵����ȷ���ǣ�������
| A������������һ������Al������������ȷ�� | B�����������п��ܺ���MgCl2��AlCl3 | C������������һ������MgCl2��FeCl2 | D������������һ�����У�NH4��2SO4��MgCl2 |