��Ŀ����
 ij��ɫ��Һ��ֻ����NH4+��K+ Al3+ Cu2+��Mg2+��CO32-��SO42-�������еļ��֣���1��ȡ100mL����Һ���μ�����ϡ�����ữ��Ba��NO3��2��Һ�����˵õ�0.03mol��ɫ����
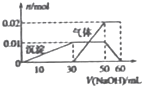
ij��ɫ��Һ��ֻ����NH4+��K+ Al3+ Cu2+��Mg2+��CO32-��SO42-�������еļ��֣���1��ȡ100mL����Һ���μ�����ϡ�����ữ��Ba��NO3��2��Һ�����˵õ�0.03mol��ɫ������2����ȡ10mL����Һ���Թ��У���μ���NaOH��Һ������ɫ���������������ӵ�һ������ʼ�������壨��Ҫʱ�ɼ��ȣ�����������ȫ�ܽ⣮��������������ʵ���������NaOH��Һ����ı仯��ϵ��ͼ��ʾ��������
| A����Һ��һ��������Cu2+��Mg2+��CO32-���ܺ���K+ | B��NH4+��Al3+��SO42-�������ӵ����ʵ���֮��Ϊ2��1��3 | C��ʵ�������õ�NaOH��Һ�����ʵ���Ũ��Ϊ0.1mol/L | D��ֻ��ͨ����ɫ��Ӧʵ����ȷ�ϸ���Һ���Ƿ����K+ |
������ij��ɫ��Һһ������Cu2+���ӣ�ȡ10mL����Һ���Թ��еμ�Ba��NO3��2��Һ����ϡ�����ữ����˵õ�0.3mol��ɫ���������ɳ���ΪBaSO4��˵����Һ�к���SO42-����ȡ10mL����Һ���Թ��У��μ�NaOH��Һ������ɫ���������������ӵ�һ������ʼ�������壬˵����Һ�к���NH4+����������ȫ�ܽ⣮��˵����Һ�к���Al3+��û��Mg2+��
Al3+��CO32-���ܹ��棬��û��CO32-�����ԭ��Һ��һ�����ڣ�NH4+��Al3+��SO42-��һ�������� Cu2+��Mg2+��CO32-������ͼ����������ж�Al��OH��3�Ͱ��������ʵ���������Al��OH��3�����Կɼ���NaOH��Ũ�ȣ�K+Ҫ����ͼ��������ݺ���Һ�����Լ����Ƿ��У�
Al3+��CO32-���ܹ��棬��û��CO32-�����ԭ��Һ��һ�����ڣ�NH4+��Al3+��SO42-��һ�������� Cu2+��Mg2+��CO32-������ͼ����������ж�Al��OH��3�Ͱ��������ʵ���������Al��OH��3�����Կɼ���NaOH��Ũ�ȣ�K+Ҫ����ͼ��������ݺ���Һ�����Լ����Ƿ��У�
����⣺ij��ɫ��Һһ������Cu2+���ӣ�ȡ10mL����Һ���Թ��еμ�Ba��NO3��2��Һ����ϡ�����ữ����˵õ�0.3mol��ɫ���������ɳ���ΪBaSO4��˵����Һ�к���SO42-�����ʵ���Ϊ0.03mol����ȡ10mL����Һ���Թ��У��μ�NaOH��Һ������ɫ���������������ӵ�һ������ʼ�������壬˵����Һ�к���NH4+����������ȫ�ܽ⣮��˵����Һ�к���Al3+��û��Mg2+��Al3+��CO32-���ܹ��棬��û��CO32-�����ԭ��Һ��һ�����ڣ�NH4+��Al3+��SO42-��һ�������� Cu2+��Mg2+��CO32-�����ܺ�K+��
��ȡ10mL����Һ���Թ��У��μ�NaOH��Һ������ɫ��������˵����Һ�к���Al3+��Al3++3OH-=Al��OH��3�������ͼ���г����ı仯��ϵ��֪��Һ�к���Al3+Ϊ0.01mol��OH-Ϊ0.03mol�����������ӵ�һ������ʼ�������壬����NH4++OH-=NH3��+H2O���ͼ��֪��Һ�к���NH4+Ϊ0.02mol��OH-Ϊ0.02mol����������ȫ�ܽ�����������������NaOH������Ӧ����Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O�����ͼ���г����ı仯��ϵ����OH-Ϊ0.01mol��ʵ����ʹ�õ�NaOH��Һ�������ʵ���Ϊ��0.03mol+0.02mol+0.01mol=0.06mol�����ͼ���г����ı仯��ϵ֪��ʱ����������Һ�����Ϊ60mL������C=
=
=lmol/L�������Ϸ�����֪NH4+Ϊ0.02mol��Al3+Ϊ0.01mol��SO42-Ϊ0.3mol��������Һ�ʵ�������Һ�����������������������������ȣ��������������Ϊ��0.02mol��1+0.01mol��3+n��K+����1=0.05mol+n��K+�������������0.03mol��2=0.06mol������n��K+��=0.01mol����һ������K+��
A���������Ϸ�����֪��һ����K+����A����
B���������Ϸ�����֪��NH4+Ϊ0.02mol��Al3+Ϊ0.01mol��SO42-Ϊ0.3mol��NH4+��Al3+��SO42-�������ӵ����� ����֮��Ϊ2��1��3����B��ȷ��
C..�������Ϸ�����֪���������Ƶ�Ũ��Ϊ1mol/L����C����
D..�������Ϸ�����֪��K+��ͨ������ȷ������D����
��ѡB��
��ȡ10mL����Һ���Թ��У��μ�NaOH��Һ������ɫ��������˵����Һ�к���Al3+��Al3++3OH-=Al��OH��3�������ͼ���г����ı仯��ϵ��֪��Һ�к���Al3+Ϊ0.01mol��OH-Ϊ0.03mol�����������ӵ�һ������ʼ�������壬����NH4++OH-=NH3��+H2O���ͼ��֪��Һ�к���NH4+Ϊ0.02mol��OH-Ϊ0.02mol����������ȫ�ܽ�����������������NaOH������Ӧ����Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O�����ͼ���г����ı仯��ϵ����OH-Ϊ0.01mol��ʵ����ʹ�õ�NaOH��Һ�������ʵ���Ϊ��0.03mol+0.02mol+0.01mol=0.06mol�����ͼ���г����ı仯��ϵ֪��ʱ����������Һ�����Ϊ60mL������C=
| n |
| V |
| 0.06mol |
| 0.06L |
A���������Ϸ�����֪��һ����K+����A����
B���������Ϸ�����֪��NH4+Ϊ0.02mol��Al3+Ϊ0.01mol��SO42-Ϊ0.3mol��NH4+��Al3+��SO42-�������ӵ����� ����֮��Ϊ2��1��3����B��ȷ��
C..�������Ϸ�����֪���������Ƶ�Ũ��Ϊ1mol/L����C����
D..�������Ϸ�����֪��K+��ͨ������ȷ������D����
��ѡB��
���������⿼�����ӵ��ƶϣ���������ṩ��ʵ�����������ӵ����ʺ�ͼ�����ݽ����ƶϼ��㣬��Ŀ�ѶȽϴ�Ҫ������õ���غ㣮

��ϰ��ϵ�д�
�����Ŀ
��������ʵ�飬����ʵ����ʵ�ó��Ľ��ۣ���ȷ���ǣ�������
| A����ij��Һ�еμ�BaCl2��Һ�ð�ɫ������ȡ�ð�ɫ������ϡ����ܽ�--ԭδ֪��Һ��һ������SO42- | B����ijδ֪��Һ�м���Ũ��NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����������--ԭδ֪��Һ��һ������NH4+ | C����ijδ֪��Һ�еμ����������ɫ���壬��������ͨ����������ʯ��ˮ�еð�ɫ����--ԭδ֪��Һ��һ������CO32- | D���ýྻ�IJ�˿պȡδ֪���ʣ�������ɫ���������գ�����ɫΪ��ɫ--��δ֪����һ��ֻ����Ԫ�� |